Credit: Pixabay/CC0 Public Domain
Cancer is a group of diseases involving abnormal cell growth with the potential to spread throughout the body. About 19.3 million new cancer cases and 10 million cancer-related deaths were reported worldwide in 2020. More than 200 different types of cancer have been identified, of which each is diagnosed and treated in a particular way. Unfortunately, no effective treatment is available to all cancer types, and the most important unmet medical need is the lack of specific targeted therapies.
Cancer is a result of the uncontrolled growth of cells, which is caused by the deregulation of multiple cellular pathways that are involved in normal growth control. Numerous studies highlight the importance of the enzyme complex called Protein phosphatase 2A (PP2A) in the inhibition of the transformation of normal human cells into cancerous cells.
The PP2A activity is inhibited by different proteins such as ARPP-16, ARPP-19, and ENSA. These proteins have been shown to play role in several cancer types such as hepatocellular carcinoma, human glioma, acute myeloid carcinoma, gastric cancer, breast cancer, and papillary thyroid cancers. Therapeutically, it is tempting to reactivate PP2A by inhibiting the interaction of PP2A and PP2A inhibitor proteins by using small molecules. For that, it is essential to understand the molecular and structural mechanism of PP2A inhibition by inhibitor proteins such as ARPP-16, ARPP-19, and ENSA.
Understanding the inhibitory mechanism
The ultimate aim of this study was to reveal the mechanism of how ARPP-16, ARPP-19, and ENSA proteins inhibit PP2A function. Understanding the inhibitory mechanism is crucial to develop small molecule modulators that can treat cancer types linked to these proteins. Considering that, we first integrated the modern structural biology methods such as NMR spectroscopy and small-angle X-ray scattering methods to investigate so far unknown structural properties of the PP2A inhibitor proteins ARPP-19, ARPP-16, and ENSA.
The results show that the ARPP and ENSA proteins lack the defined three-dimensional structure being intrinsically disordered proteins but have propensities to form transient secondary structures. Both ARPPs and ENSA have three short transient helical regions and these preformed structures can act as recognition elements during interaction with their partner. Intrinsic disorder proteins are multifaceted and manifest themselves in various forms that allow them to exert different functions in a different cellular context.
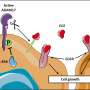
We used microscale thermophoresis and NMR spectroscopy techniques to understand the interaction mechanism between the PP2A and ARPPs/ENSA. The interaction studies showed that ARPP and ENSA proteins interact differently with different PP2A subunits. ARPP/ENSA proteins bind to PP2A A-subunit with modest affinity, whereas the interaction to the regulatory B-subunit is weak and transient.
Our studies also revealed that ARPPs and ENSA utilize different regions in their interaction with PP2A scaffolding A-subunit; ARPPs bind to A-subunit via a linear motif comprising the second transient α-helical region, whereas ENSA binds to PP2A A-subunit using extended binding region that comprise all three transient α-helical regions.
Accordingly, both ARPPs and ENSA seem to utilize a two-step mechanism in the binding with PP2A. In the first step, ARPPs/ENSA binds to the target site on the A-subunit of PP2A. In the next step, the interaction of ARPPs/ENSA to the B-subunit of PP2A is guided by the local interaction with the target site at A-subunit. Although our results do not give a complete molecular and structural level explanation for the ARPP mediated PP2A inhibition, our results provide a good starting point for future studies and the development of therapeutics that block ARPPs/ENSA—PP2A interaction and reactive PP2A.
More information: Structural insight of PP2A inhibitor proteins and their interaction with PP2A A- and B56-subunit. urn.fi/URN:ISBN:978-951-39-8486-1
Provided by University of Jyväskylä