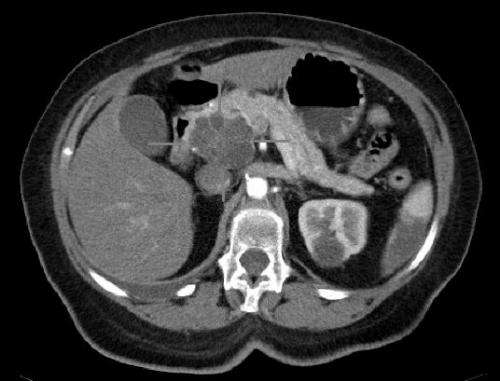
Axial CT image with i.v. contrast. Macrocystic adenocarcinoma of the pancreatic head. Credit: public domain
Pancreatic cancer, one of the most lethal of all cancers, is capable of evading attacks by immune cells by changing its microenvironment so that the immune cells suppress, rather than support, an attack on the tumor. The scientists also found that that some of the mediators of this suppressive response, including a protein called STAT1, represent potential therapeutic targets that could be used to reverse this evasion and point to possible treatment opportunities.
The finding appears January 28, 2021, in Cancer Immunology Research.
"This is the first demonstration that an immune attack induces pancreatic cancer-derived immune suppression, offering a new approach to immunotherapy for this deadly cancer," noted Louis M. Weiner, M.D., director of Georgetown Lombardi and the Principal Investigator of this study.
Pancreatic ductal adenocarcinoma (PDAC) comprises over 90 percent of all pancreatic cancers, with nearly 60,000 diagnoses expected in the U.S. in 2021. Only 10 percent of those diagnosed will live five years or more, primarily because the cancer is highly resistant to many types of treatments. Despite major recent advances in cancer immunotherapy, this cancer rarely responds to such treatment approaches which have revolutionized the management of other cancers, such as melanoma and lung cancer.
One reason for this treatment resistance is the tumor microenvironment of PDAC, which actively suppresses immune responses that are helpful in attacking cancer cells. Reham Ajina, Ph.D., a recent graduate of the Georgetown University Medical Center's Tumor Biology Program, studied mice to explore how the immune cell that is most responsible for recognizing and killing cancer cells, the T cell, is able to elicit an anti-tumor T-cell response in many cancers, but not in most pancreatic cancers.
"Tumor tissue is comprised of not only cancer cells, but also a wide range of non-cancerous elements, such as immune, fat, and neuronal cells, along with fibers and blood vessels that comprise the tumor microenvironment," said Ajina. "Normally, T cells recognize and kill cancer cells, but it appears that malignant pancreatic cells are simultaneously trying to find ways to evade a T cell immune attack by influencing components of the tumor microenvironment to favor cancer development and growth, a process called remodeling. Inhibiting this remodeling is a major challenge in trying to treat pancreatic cancer."
One of the keys to being able to visualize this remodeling in mice was made possible by cutting-edge analytical technologies contributed by collaborators at Johns Hopkins University and the Oak Ridge National Laboratory.
Beyond the finding of remodeling and evasion, the research team was able to determine that one of the mediators of this suppressive response included an activated protein called signal transducer and activator of transcription 1 (STAT1). The researchers hypothesized that STAT1-based signaling could be targeted to reverse this resistance mechanism. The investigators chose an FDA-approved drug, ruxolitinib, that is known to target a STAT signaling pathway, to test in mice. Indeed, use of the drug overcame tumor-protective remodeling responses and helped improve the response to immunotherapy.
"Fludarabine is another FDA-approved drug that targets STAT1 signaling, and there are several other similar-acting drugs that are currently under development. It would be worth testing the therapeutic benefit of these drugs in future studies to evaluate and corroborate our observation," said Ajina. "Also, our pre-clinical study in mice suggests that the combination of ruxolitinib and other approved immunotherapies could improve pancreatic cancer patients' outcomes. This approach to treating an aggressive cancer is promising and we hope that it can be tested in clinical trials in the not-too-distant future."
More information: Louis M Weiner et al, Antitumor T-cell Immunity Contributes to Pancreatic Cancer Immune Resistance Cancer Immunol Res January 28 2021 DOI: 10.1158/2326-6066.CIR-20-0272
Provided by Georgetown University Medical Center