Stomach-specific c-Myc overexpression and gastric cancer
Gastric cancer is one of the most common malignant cancers worldwide. Since the stage of early gastric cancer usually lasts for a long time and lacks obvious symptoms, the diagnosis of early gastric cancer is very difficult. Therefore, gastric cancer patients typically have a poor prognosis and low survival rate. During the progression of gastric cancer, genetic factors play an indispensable role. c-Myc, an oncogene, is abnormally highly expressed in a variety of tumors, including gastric cancer. For example, the Hi-Myc mouse model with high expression of c-Myc has been widely used in prostate cancer research. However, there is still no gastric cancer mouse model with increased expression of c-Myc, and it is unclear how c-Myc promotes gastric cancer in the early stage.
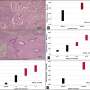
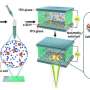
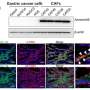
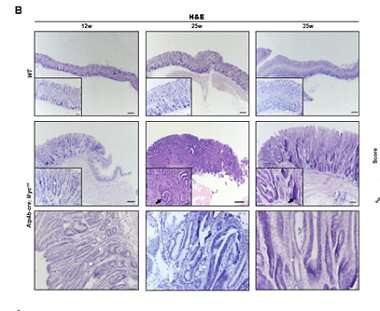
In the study published in the Bosnian Journal of Basic Medical Sciences, the authors successfully generated a stomach-specific c-Myc transgenic mouse model. They found that overexpression of c-Myc in Atp4b+ gastric parietal cells could induce gastric adenoma in mice. Mechanistically, c-Myc promoted tumorigenesis via the AKT/mTOR pathway. Furthermore, AKT inhibitor (MK-2206) or mTOR inhibitor (Rapamycin) repressed the proliferation of c-Myc overexpressing gastric cancer cells and the gastric tumorigenesis in c-Myc transgenic mice.
In general, the authors of the study first established a stomach-specific c-Myc transgenic mouse model, which provided a brand-new animal model for subsequent gastric cancer research. Also, their findings highlight that gastric tumorigenesis induced by c-Myc overexpression is through activation of the AKT/mTOR pathway.
More importantly, their research is of important clinical significance for the diagnosis and molecular targeted therapy of early gastric cancer.
More information: Jing Liu et al. Stomach-specific c-Myc overexpression drives gastric adenoma in mice via AKT/mTOR signaling, Bosnian Journal of Basic Medical Sciences (2020). DOI: 10.17305/bjbms.2020.4978