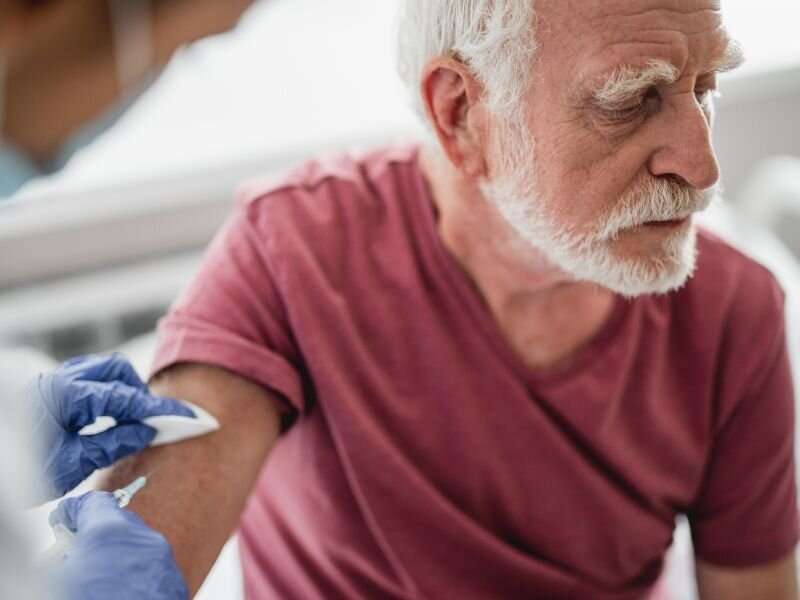
Even though 11.4 million doses of the approved Pfizer and Moderna COVID-19 vaccines had been distributed across the United States by Monday morning, just 2.1 million had made it into the arms of high-risk Americans.
That's far too slow a pace, said one official charged with spearheading the vaccination of Americans.
"We agree that that number is lower than what we hoped for," Moncef Slaoui, scientific adviser of Operation Warp Speed, the federal effort to accelerate vaccine development and distribution, said at a Wednesday news briefing, The New York Times reported. "We know that it should be better," he said, "and we're working hard to make it better."
Already by Wednesday, the number of distributed doses had risen to 14 million, and the 2.1 million vaccination tally—compiled by the U.S. Centers for Disease Control and Prevention—could be somewhat low. At a separate news briefing held Wednesday, the CDC announced the number of Americans who'd gotten the first dose of vaccine stood at 2.6 million, the Times said.
Still, even that number is a far cry from an earlier prediction federal officials had made that 20 million people would have gotten a dose of vaccine by the end of December.
Just why delays are happening is unclear.
Speaking at the "Warp Speed" news briefing, logistics coordinator Gen. Gustave Perna cited possible reasons as the holiday season, winter weather and lags in reporting as possible factors slowing delivery. According to the Times, he said that health care facilities are still learning how to store the vaccines at super-low temperatures, and many states are setting aside doses for use at long-term care facilities, an effort that's expected to take several months.
Right now, most shots are being given at hospitals, clinics and nursing homes, but Perna and Slaoui agreed that the pace of vaccination should pick up considerably once doses are given out by the major pharmacy chains.
"What we should be looking at is the rate of acceleration over the coming weeks," Slaoui said, "and I hope it will be in the right direction."
President-Elect Joe Biden has been critical of the slow pace of vaccine deployment. Speaking Tuesday in Wilmington, Del., he said that at current rates, "it's going to take years, not months," to vaccinate the entire U.S. population.
Biden is vowing that upon taking office on Jan. 20, he will he activate a law known as the Defense Production Act to "order private industry to accelerate the making of the materials needed for the vaccines as well as protective gear."
But the Trump administration has already taken that step to speed up vaccine manufacturing, the Times noted, so it's unclear how Biden's plan will differ. Biden has pledged to administer 100 million vaccine doses—enough to provide 50 million people with the two doses needed for protection—within the first 100 days of his presidency.
"This is going to be the greatest operational challenge we've ever faced as a nation," Biden said, "but we're going to get it done."
New coronavirus variant now spotted in California, Colorado
California announced the nation's second case of the new and more contagious variant of the coronavirus, the Associated Press reported Wednesday.
In an online conference with Dr. Anthony Fauci, head of the U.S. National Institute of Allergy and Infectious Diseases, Gov. Gavin Newsom announced the infection was found in Southern California.
"I don't think Californians should think that this is odd. It's to be expected," Fauci said.
The announcement came a day after the first case was reported in Colorado.
Experts believe the new variant is probably already spreading elsewhere in the United States.
"The virus is becoming more fit, and we're like a deer in the headlights," Dr. Eric Topol, head of Scripps Research Translational Institute, told the AP.
Topol said that the United States does less genetic sequencing of virus to discover variants than other nations, and thus was probably slow to detect this new mutation.
Other states, including California, Massachusetts and Delaware, are analyzing suspicious virus samples for the variant, Dr. Greg Armstrong, who directs genetic sequencing at the CDC, told the AP.
No evidence has been found that this variant is more deadly or causes more severe illness, and scientists are saying that the vaccines will be effective against it. But a faster-spreading virus could swamp hospitals with seriously ill patients.
Researchers estimate the variant is 50% to 70% more contagious, Dr. Eric France, Colorado's chief medical officer, told the AP.
"Instead of only making two or three other people sick, you might actually spread it to four or five people," France said. "That means we'll have more cases in our communities. Those number of cases will rise quickly and, of course, with more cases come more hospitalizations."
The rapid spread of the new variant within Britain has caused a virtual shutdown there, with many countries banning or restricting flights from the United Kingdom. Many scientists in the United States had assumed that the novel variant was already circulating among Americans.
Another COVID-19 vaccine enters final trials
In other news, vaccine maker Novavax, along with federal health researchers, announced Monday that a phase 3 trial will begin on the safety and effectiveness of another COVID-19 vaccine—the fifth shot to reach this final stage of development.
"We've come this far, this fast, but we need to get to the finish line," Dr. Francis Collins, director of the U.S. National Institutes of Health (NIH), said in an NIH statement.
Novavax will enroll 30,000 people from 115 testing sites across the United States and Mexico, and testing is already underway in Britain. The vaccine—which right now is known as NVX-CoV2373—comes in two doses and is designed to enhance the body's immune response to the coronavirus' distinctive spike protein.
The Novavax shot is somewhat different from approved vaccines from Pfizer and Moderna, in that it manufactures its own antigens that mimic the coronavirus' spike protein. However, these antigens "cannot replicate and cannot cause COVID-19," the NIH said in the statement.
If phase 3 trials prove the Novavax vaccine to be safe and effective, the shot has one big advantage over the Moderna and Pfizer vaccines: It needs only standard refrigeration, not the freezing or ultra-cold temperature storage required by the first two vaccines.
According to CBS News, two other pharmaceutical companies, Johnson & Johnson's Janssen and AstraZeneca, also have phase 3 COVID-19 vaccine trials underway in the United States.
A global scourge
By Thursday, the U.S. coronavirus case count passed 19.7 million while the death toll neared 343,000, according to a Times tally. On Thursday, the top five states for coronavirus infections were: California with nearly 2.3 million cases; Texas with almost 1.8 million cases; Florida with 1.3 million cases; New York with almost 964,000 cases; and Illinois with nearly 958,000 cases.
Curbing the spread of the coronavirus in the rest of the world remains challenging.
In India, the coronavirus case count was over 10.2 million on Thursday, a Johns Hopkins University tally showed. Brazil had over 7.6 million cases and close to 194,000 deaths as of Thursday, the Hopkins tally showed.
Worldwide, the number of reported infections neared 83 million on Thursday, with more than 1.8 million deaths recorded, according to the Hopkins tally.
More information: The U.S. Centers for Disease Control and Prevention has more on the new coronavirus.
Copyright © 2020 HealthDay. All rights reserved.