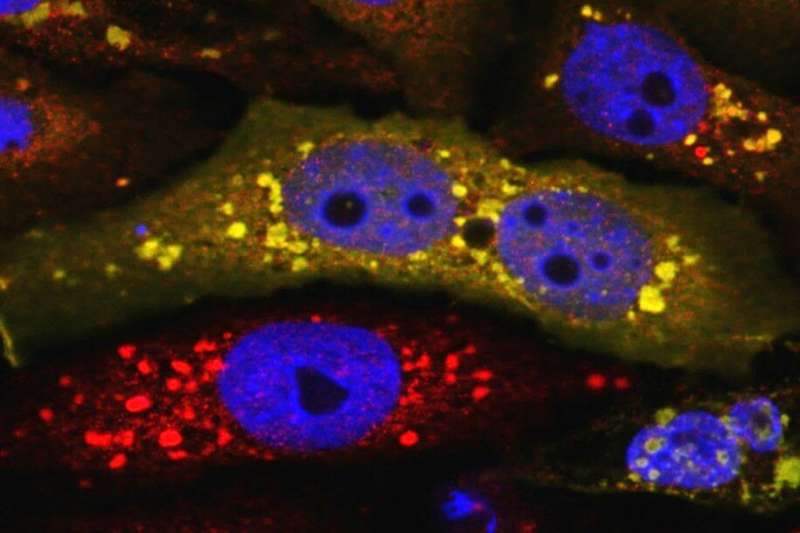
The insoluble form of p62 protein is abundant in liver cancer cells where it causes hyper-activation of the cancer-promoting Nrf2 signalling pathway. The image shows the recruitment of the MOAP-1 protein at the insoluble p62 protein aggregates in the liver cancer cells. The MOAP‐1 protein (yellow/orange) is co-located with the p62 aggregates (red) surrounding the nuclei (blue) of the liver cancer cells. Credit: Chong Teik TAN
National University of Singapore researchers have discovered that the MOAP-1 protein plays a role in reducing liver cancer risk by suppressing the hyper-active cancer-promoting Nrf2 cellular signaling pathway.
Hepatocellular carcinoma (HCC), the primary form of liver cancer, is the fourth leading cause of cancer death worldwide. Although many risk factors and genetic aberrations have been identified for HCC, its prognosis remains highly unfavorable due to limited therapeutic options. The symptoms associated with liver cancer do not usually appear until later stages, which further contribute to poor patient survival rates. Thus, understanding the development of HCC at the molecular level is an important step for gaining new insights to facilitate the identification of potential drug targets to treat this disease.
A team led by Prof Victor YU from the Department of Pharmacy, NUS discovered that the MOAP-1 protein reduces the amount of insoluble p62 protein aggregates in the liver. This effectively suppresses the excessive activation of a cellular signaling pathway known as the nuclear factor erythroid 2-related factor 2 (Nrf2). High levels of the p62 protein can accumulate and form insoluble aggregates in our liver to cause hyper-activation of the Nrf2 signaling pathway, which is in turn known to enhance the fitness of the cancer-initiating cells, causing them to spread and possibly evade the human body's immune system. Therefore, high levels of the p62 protein are strongly associated with the occurrence and poor prognosis of liver cancer.
The research team found that the MOAP-1 protein physically interacts with the p62 protein, interfering with its self-oligomerisation process that is known to play a pivotal role in causing p62 aggregation into an insoluble form. This causes the p62 protein aggregates to disassemble, which in turn results in dampening their ability to stimulate the Nrf2 signaling. The research uncovers an important regulatory step that can be explored further to suppress the tumourigenic signaling mechanism associated with HCC development. The MOAP-1 protein is regulated by an enzyme-linked mechanism that controls its protein stability. Through development of small molecular compounds that can manipulate this mechanism, it is feasible that the amount of MOAP-1 protein can be elevated in the liver to exert a tumor suppressive-like effect, rendering it a potential drug target candidate for the treatment of HCC.
Moving forward, the team will also be embarking on investigating the role of MOAP-1 in the development of non-alcoholic fatty liver disease (NAFLD), which is a rapidly emerging cause of HCC worldwide including Singapore.
Prof Yu added, "NAFLD encompasses a spectrum of liver disorders, from steatosis to non-alcoholic steatohepatitis that can progress to life-threatening conditions including cirrhosis and HCC. Currently, there is a lack of effective treatments for NAFLD. The p62 protein is known to play a role in NAFLD. However, details on how it can be controlled remain poorly understood. Our current discovery of the MOAP-1 as a regulator of the p62 protein is expected to open up new research avenues to investigate its potential role in the treatment of NAFLD and HCC."
More information: Chong Teik Tan et al. MOAP‐1‐mediated dissociation of p62/SQSTM1 bodies releases Keap1 and suppresses Nrf2 signaling, EMBO reports (2021). DOI: 10.15252/embr.202050854
Journal information: EMBO Reports
Provided by National University of Singapore