A new vision for AAV-delivered gene therapies
In recent years, adeno-associated virus (AAV) has been recognized as the leading vehicle (vector) for in vivo delivery of therapeutic genes because it is non-pathogenic and efficiently targets many different cell and tissue types. The recent Federal Drug Administration (FDA) approvals of AAV-based gene-replacement therapies to treat spinal muscular atrophy and a form of inherited retinal dystrophy highlight the promise of this therapeutic modality.
A key challenge of in vivo gene therapies is their potential to cause immune reactions and inflammation, which can affect how well the therapies work or last, and in rare cases can even be life-threatening. The recently reported deaths of three children that received a high-dose systemically delivered AAV gene therapy in a trial to treat X-linked myotubular myopathy dramatically showed that AAV-mediated toxicity and immune responses are only incompletely understood and that current AAV delivery vehicles still need to be further improved.
The AAV capsid and genome can both act as immunogenic components. Specifically, the vector genome, which encompasses the therapeutic gene, can activate a protein known as Toll-like receptor 9 (TLR9), a so-called pattern recognition receptor that senses foreign DNA in specialized immune cells. This sensing first triggers an immune response that results in inflammation (innate immunity), and subsequently more specific immune responses (adaptive immunity, in the form of cytotoxic T cells) against the AAV capsid, preventing the therapy from taking effect and posing a potential risk.
Now, an international collaboration of leading groups in gene therapy and vision science, including George Church's group at Harvard's Wyss Institute for Biologically Inspired Engineering and Harvard Medical School (HMS) and Constance Cepko's group at HMS, developed a "coupled immunomodulation" strategy in which short TLR9-inhibitory sequences are incorporated directly into the much longer AAV genome containing therapeutic DNA sequences. Investigated in different tissues of mice, as well as ocular tissues of pigs and non-human primates, the approach showed broad anti-immunogenic potential. Importantly, the study also highlights that pathways other than TLR9 activation likely contribute to inflammation in the highly immunogenic model of intravitreal AAV injections in macaques. The work is published in Science Translational Medicine.
The project was initiated in George Church's group at the Wyss Institute and HMS. Church, Ph.D., is a Core Faculty member at the Wyss Institute and leads the Institute's Synthetic Biology platform. He also is Professor of Genetics at Harvard Medical School and of Health Sciences and Technology at Harvard and the Massachusetts Institutes of Technology (MIT).
Cloaking AAV using coupled immunomodulation
"We hypothesized that small snippets of DNA that bind and inhibit TLR9 activation, including DNA sequences from the ends of human chromosomes called telomeres, would be a way to cloak the AAV genome from this immune-surveillance mechanism when incorporated directly into it," said first- and co-corresponding author Ying Kai Chan, Ph.D., formerly a postdoctoral fellow working with Church and Chief Scientific Officer at Ally Therapeutics, and currently a visiting scholar at the Wyss. Chan and Church are both co-founders of Wyss gene therapy startup company Ally Therapeutics.
The team started by generating a series of synthetic DNA "inflammation-inhibiting oligonucleotide" (IO) sequences that each carry a highly inflammatory portion linked to one of different TLR9-inhibitory sequences, and tested their effects on cultured cells. The presence of TLR9-inhibitory sequences dampened the inflammatory response by up to 95%. When directly incorporated as a tandem series into an AAV vector, the IOs dampened innate immune responses in primary human immune cells compared to an unmodified vector
To test the strategy in AAV in vivo, the researchers administered AAVs as a systemic treatment or locally into muscle tissue of mice. Control viruses lacking IO sequences induced anti-viral interferon responses and the infiltration of innate immune cells in livers, and led to infiltration and activation of cytotoxic T cells in muscle tissues. These effects were absent in mutant mice lacking a functional TLR9 pathway, showing that TLR9 was indeed a key regulator of AAV-induced inflammation. Importantly, the effects were blocked or much reduced in mice that received engineered AAVs containing IO sequences in their genomes, and the coupled immunomodulation strategy enhanced expression of the transgene that the virus delivered, indicative of potentially higher efficacy.
Investigating coupled immunomodulation in the eye
The eye is often described as an immune-privileged site because of the presence of a blood-retina barrier that limits entry of immune cells, and of immune-suppressive factors. However, multiple clinical trials have reported intraocular inflammation following delivery of therapeutically relevant doses of AAV into the eye, demonstrating a limit for immune privilege. Most AAV-based gene therapies in the eye are directly applied to the retina (subretinal injection). AAV delivery to the vitreous cavity (intravitreal injection) of the eye is highly desirable since it would be less invasive and potentially allows for targeting more cells, but it is unfortunately highly inflammatory.
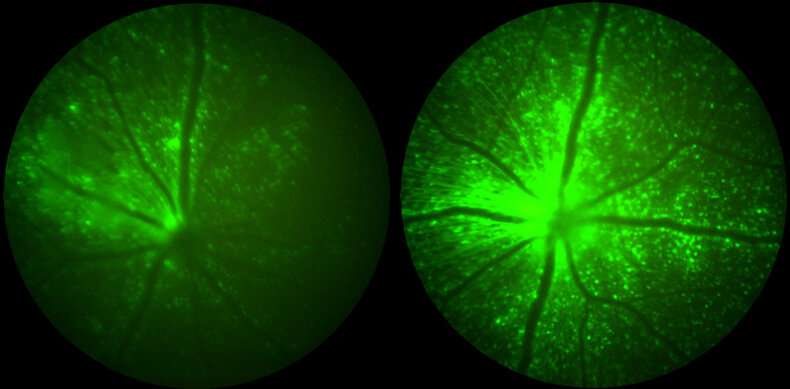
Using in vivo imaging and immune cell characterization techniques after intravitreal injection of AAV virus in mice, the team demonstrated that the incorporation of IO sequences in the virus genome reduced the inflammation and numbers of infiltrating T cell populations in the eye compared to unmodified AAVs. This further coincided with a multifold boost in expression of the vector-encoded reporter gene in the retina.
Next, the team studied their coupled immunomodulation strategy in large animal models, first in pigs via subretinal injections, and then in macaque monkeys via intravitreal injections. "We found that the strategy ameliorated distinct pathologies triggered by control AAV viruses in pigs, including the shortening of photoreceptor cells essential for high-acuity vision," said Sean Wang, M.D., who collaborated with Chan as a medical student in Cepko's group. They also found the infiltration of the photoreceptor layer of the retina by immune cells, including microglia and T cells, to be substantially rescued.
"In macaques that received engineered and control AAVs via intravitreal injections, these immunosuppressive effects unfortunately were not as pronounced although we saw that the coupled immunomodulation approach delayed the clinical uveitis symptoms triggered by control virus, and allowed a two-fold increase in the expression of a therapeutic gene," said Wang. Also, the use of prophylactic systemic immunosuppression was unable to prevent the observed uveitis, showing that immunogenicity challenges for this route are more complex.
"The results from the intravitreal toxicity induced by AAV, and the modest response to the TLR9 blocking sequence and to steroids, indicate that there is more than one mechanism leading to toxicity from this injection site. We can now go forward with this understanding and search for additional pathways," said Cepko, Ph.D., the Bullard Professor of Genetics and Neuroscience in the Blavatnik Institute at HMS, and an Investigator of the Howard Hughes Medical Institute and member of the Harvard Stem Cell Institute.
"Every novel therapeutic modality that achieves initial success in the clinic has to grapple with emerging issues before it can be deployed broadly, and AAV gene therapy is no exception. Our work represents a critical step in development of next generation AAV vehicles that are safer and more effective" said Chan.
"This important Wyss-initiated collaboration among leading experts in synthetic biology, vision science and gene therapy opens up a new direction in AAV engineering that ultimately could help make gene therapies safer and more effective through targeted viral genome engineering," said Wyss Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
More information: Y.K. Chan el al., "Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses," Science Translational Medicine (2021). stm.sciencemag.org/lookup/doi/ … scitranslmed.abd3438