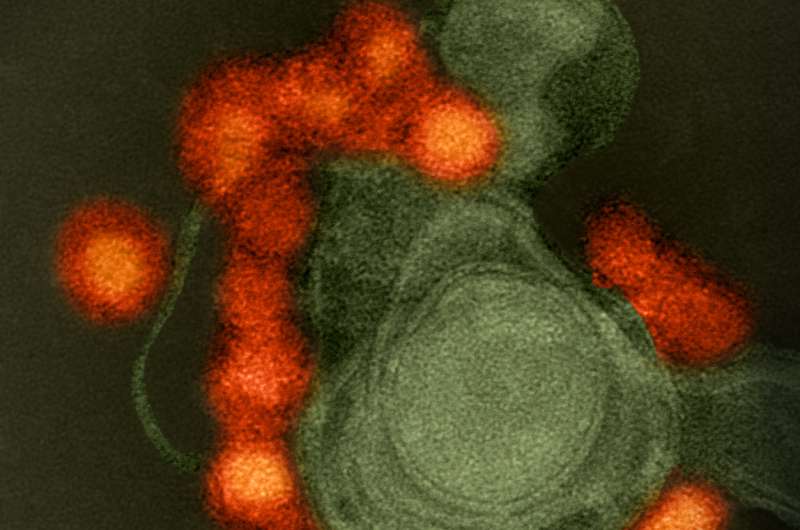
Transmission electron microscope image of negative-stained, Fortaleza-strain Zika virus (red), isolated from a microcephaly case in Brazil. The virus is associated with cellular membranes in the center. Credit: NIAID
The Zika virus candidate, Ad26.ZIKV.001, a replication-incompetent human adenovirus serotype 26 (ad26) vector showed promising safety and immunogenicity in a phase I clinical trial. Researchers say the vaccine warrants further development should the need reemerge. The findings are published in Annals of Internal Medicine.
Zika virus (ZIKV) infection is transmitted via mosquito or sexually and may cause severe congenital disease after maternal-fetal transmission. The incidence of Zika virus has declined since the 2015-2016 outbreak, but geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics. Currently, no vaccine is available.
Researchers from Janssen Vaccines and Prevention and Beth Israel Deaconess Medical Center randomly assigned 100 healthy participants to either a 1- or 2-dose regimen of Ad26.ZIKV.001 or placebo to assess the safety and immunogenicity of the Zika vaccine candidate. They found that 2 doses of Ad26.ZIKV.001 were safe, caused mild to moderate reactogenicity, and induced persistent neutralizing antibody responses.
Transferred antibodies were found to be protective in a mouse Zika challenge model. Antibody responses up to 1 year after vaccination were observed in at least 80% of participants in both 2-dose (high and low) groups, indicating that a low dose would be sufficient. A single-dose vaccine had lower peak neutralizing antibody responses than the 2-dose strategies but nevertheless showed durable antibody titers at 1 year, and thus may be a useful tool in curbing future Zika epidemics.
More information: Abstract: https://www.acpjournals.org/doi/10.7326/M20-5306
Editorial: https://www.acpjournals.org/doi/10.7326/M21-0397
Journal information: Annals of Internal Medicine
Provided by American College of Physicians