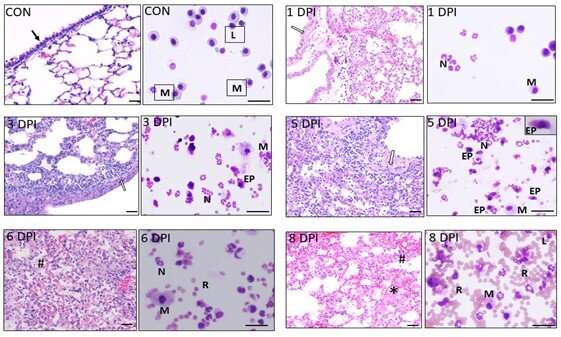
These findings provide important clues regarding the inflammatory cellular signature at different time intervals during severe influenza infection. Temporal dynamics in hematoxylin and eosin-stained lung sections or modified Geimsa-stained bronchoalveolar lavage cells from control and lethal influenza-challenged mice were evaluated at 1, 3, 5, 6, and 8 dpi. Initial epithelial cytopathic effects were prominently seen at 1 dpi (open arrow), which spread to alveoli and were followed by the severe inflammatory phase (3-5 dpi). By 6 dpi, significant vascular leakage and collapse of the alveoli became prominent, and animals died between 8-9 dpi with severe hemorrhagic exudates (hash) with abundant proteinaceous material in the alveolar spaces (asterisk). CON-Control; DPI-Days post infection; M-macrophage; L-lymphocyte; N-neutrophil, EP-epithelial cell. Representative image was selected from 5 mice per group. Credit: The American Journal of Pathology
A significant proportion of hospitalized patients with influenza develop complications of acute respiratory distress syndrome, driven by virus-induced cytopathic effects as well as exaggerated host immune response. Reporting in The American Journal of Pathology, investigators have found that treatment with an immune receptor blocker in combination with an antiviral agent markedly improves survival of mice infected with lethal influenza and reduces lung pathology in swine-influenza-infected piglets. Their research also provides insights into the optimal timing of treatment to prevent acute lung injury.
Previously, the investigators found that an excessive influx of neutrophils, infection fighting immune cells, and the networks they create to kill pathogens, known as neutrophil extracellular traps (NETs), contribute to acute lung injury in influenza infection. Formation of NETs by activated neutrophils occurs via a cell death mechanism called NETosis and the released NETs contain chromatin fibers that harbor toxic components.
A mouse model, commonly used in exploring influenza pathophysiology and drug therapies, was used in the current study. Because mice are not natural hosts for influenza, further validation in larger animals is necessary before testing in humans. Therefore, researchers also tested piglets infected with swine influenza virus. The animals were treated with a combination of a CXCR2 antagonist, SCH527123, together with an antiviral agent, oseltamivir.
The combination of SCH527123 and oseltamivir significantly improved survival in mice compared to either of the drugs administered alone. The combination therapy also reduced pulmonary pathology in piglets.
"Combination therapy reduces lung inflammation, alveolitis, and vascular pathology, indicating that aberrant neutrophil activation and release in NETs exacerbate pulmonary pathology in severe influenza," explains lead investigator Narasaraju Teluguakula, Ph.D., Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, OK, USA. "These findings support the evidence that antagonizing CXCR2 may alleviate lung pathology and may have significant synergistic effects with antiviral treatment to reduce influenza-associated morbidity and mortality."
It can be challenging to balance the suppression of excessive neutrophil influx without compromising the beneficial host immunity conferred by neutrophils. Therefore, the researchers examined the temporal dynamics of NETs release in correlation with pathological changes during the course of infection in mice. During the early inflammatory phase, three to five days post infection, significant neutrophil activation and NETs release with relatively few hemorrhagic lesions was observed. In the late hemorrhagic exudative phase, significant vascular injury with declining neutrophil activity was seen.
Dr. Teluguakula also emphasizes that these findings provide the first evidence to support the strategy of testing combination therapy in a large animal influenza model. "In view of the close similarities in pulmonary pathology and immune responses between swine and humans, pig-influenza pneumonia models can serve as a common platform in understanding pathophysiology and host-directed drug therapies in human influenza infections and may be useful in advancing the translational impact of drug treatment studies in human influenza infections."
More information: Harshini K. Ashar et al, Administration of a CXC Chemokine Receptor 2 (CXCR2) Antagonist, SCH527123, Together with Oseltamivir Suppresses NETosis and Protects Mice from Lethal Influenza and Piglets from Swine-Influenza Infection, The American Journal of Pathology (2021). DOI: 10.1016/j.ajpath.2020.12.013
Journal information: American Journal of Pathology
Provided by Elsevier