This lighthearted cartoon, supplied by Chi Wai Eric So, PhD., illustrates the research's key finding: the repositioning of a diarrheal drug for leukemia treatment. Credit: Emily Zeisig
New research into one of the most common and difficult cancers to treat has revealed an effective route to mitigating chemotherapy resistance through the use of a drug already approved by the FDA to treat diarrhea. The findings are published in Science Translational Medicine.
Acute myeloid leukemia (AML) is one of the most common cancers in the U.S. The disease causes approximately 23,000 deaths per year, according to the American Cancer Society, and worse, often resists chemotherapy and other frontline cancer treatments. While most patients survive more than five years, treatment is often ongoing and financially prohibitive. In order to develop effective treatments, researchers in oncology and pharmaceutical targeting need to know why AML is so resistant to treatment, and identify a route to circumvent that resistance. Speaking to Medical Xpress, Dr. Chi Wai Eric So, Ph.D., Professor and Chair in Leukemia Biology at King's College London and corresponding author of the study, explains the research: "DNA mutations are the drivers for cancer development," he says. "However, these mutations have to take place in the right cells to become cancerous. Identifying the cells of origin are important to understand cancer biology and develop better therapeutic strategy. In this study, we have reconstructed and identified the cells of origin of a highly aggressive and treatment resistant form of human leukemia."
"We discovered that these treatment-resistant leukemia cells expressed a special protein to pump out chemo-drugs," Dr. So says. That finding allowed the team to target the cause of treatment resistance right from the source. "Using an antibiotic available in the clinic for diarrheal treatment to suppress the activity of this pump, we were able to re-sensitize the cells to chemotherapy, and significantly extend the survival of mice transplanted with human leukemia cells," Dr. So explained in an email. "Let's put it this way: knowing the car that the hijacker drives is important for the police to predict his next move and stop him getting to his safehouse. Identifying the cancer cells of origins is a fundamental and important step to understand their biology and finding a potential cure."
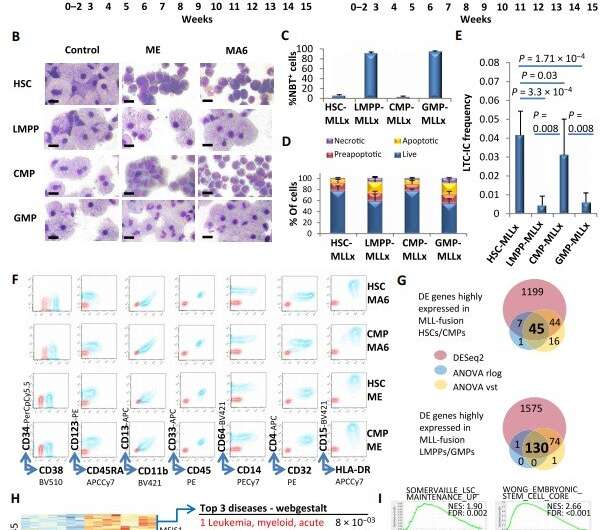
Fig. 1. Human HSCs and CMPs but not LMPPs or GMPs are cellular targets for MLL fusion–mediated transformation. (A) Proliferation of cells in culture was measured as fold expansion each week for the normal and MLL-transformed populations of cells. ME, MLL-ENL; MA6, MLL-AF6. (B) Typical cell morphology at 4 to 6 weeks of in vitro culture of the indicated cell types. Scale bar, 10 m. (C) NBT staining defining the percentage of myeloid-differentiated cells of indicated groups (n = 4 per cell of origin). MLLx: MLL-ENL and MLL-AF6. P = 1.25 × 10−19, HSC-/CMP-MLLx versus LMPP-/GMP-MLLx. (D) Bar chart represents the percentage of cells in each category after annexin V and propidium staining of the indicated cells. Data are presented as means ± SD, and statistical significance of P = 0.00319 was determined by ANOVA from N=3, n=6, F- value=14.86, df=1, for HSC-/CMP-MLLx versus LMPP-/GMP-MLLx. (E) Longterm culture initiating cell (LTC-IC) frequencies of the indicated groups at 4 weeks of in vitro culture (n = 6). (F) Typical surface marker abundance of the indicated transformed cell populations at 8 to 10 weeks in culture. (G) Venn diagram showing the overlap of significantly differentially expressed (DE) genes between HSC/CMP-MLLx and LMPP/ GMP-MLLx cells using DESeq2, ANOVA (on rlog transformed read counts) and ANOVA (on vst transformed read counts) as indicated. (H) Heatmap showing the 130 genes with consistently higher expression in LMPP/GMP-MLLx and the 45 genes with consistently higher expression in HSC/CMP-MLLx and their functional annotations according to webgestalt and Toppgene. (I) Selected stem cell–related gene sets from GSEA are shown comparing HSC-/CMP-MLLx (HC-MLLx) and LMPP-/GMP-MLLx (LG/MLLx) cells. In (A), (C), and (E), data are represented as means ± SD. In (C) and (E), statistical significance was determined by Student t test. Credit: Zeisig et al., Sci. Transl. Med. 13, eabc4822 (2021) 24 February 2021
This is promising news for AML patients and leukemia secialists alike. Dr. So is optimistic about the findings and their practical applications. "The discovery of an antibiotic with the ability to sensitize a highly aggressive and resistant form of leukemia to chemo-treatment will provide a new hope for those patients who otherwise have no treatment options. Also since this antibiotic is already in used for diarrheal, it will allow a relatively fast and easy reposition for leukemia treatment."
FDA approval of a drug is a lengthy process, but gaining approval for off-label usage—that is, a drug's effectiveness in treating a disease or illness it is not already approved to treat—is much faster, sometimes shaving decades from the gap between discovery and market use. That approval process, like all others, begins with clinical trials. Here again, Dr. So has high hopes. "In the clinical front, we hope that our findings of the potential usefulness of this antibiotic in overcoming treatment resistance will be swiftly translated into patient benefits via early phase clinical trials." But the team isn't finished, he says, even with this promising work underway. "At the same time, we are going to perform more comprehensive characterization of these leukemia cells to try to better understand their biology and to identify even better targets for combating the disease."
More information: Bernd B. Zeisig et al. Functional reconstruction of human AML reveals stem cell origin and vulnerability of treatment-resistant MLL-rearranged leukemia, Science Translational Medicine (2021). DOI: 10.1126/scitranslmed.abc4822
Journal information: Science Translational Medicine
© 2021 Science X Network