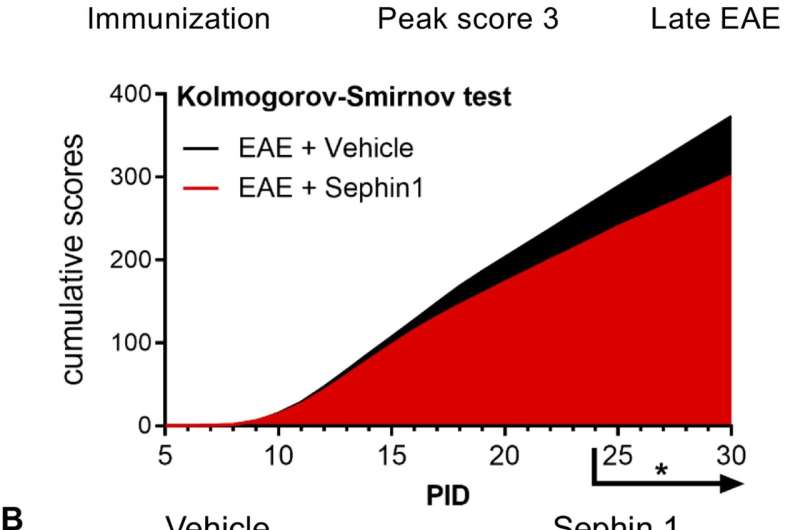
Sephin1 treatment enhances remyelination after inflammatory demyelination. (A) Cumulative clinical scores of C57BL/6J female mice immunized with MOG35-55/CFA to induce chronic EAE, treated with vehicle (n = 7) and 8 mg/kg Sephin1 (n = 7) from the peak disease. *p<0.05. Significance based on Kolmogorov-Smirnov test. (B) Representative EM images of axons in the spinal cord white matter tracts of EAE mice treated with vehicle or Sephin1 at PID30. Remyelinated axons in the EAE spinal cord were identified by thinner myelin sheaths (*). Scale bar = 1 µm. (C) Density of myelinated axons with g-ratio <0.8 in the EAE spinal cord. (D) Density of total myelinated axons in the EAE spinal cord. Data are presented as the mean ± SEM (n = 3 mice/group). Over 300 axons were analyzed per mouse. *p<0.05. Significance based on unpaired t-test. Credit: eLife (2021). DOI: 10.7554/eLife.65469
Prolonging a cellular defense response to inflammation could help regenerate the protective coating of axons that is degraded in diseases such as multiple sclerosis (MS), according to a Northwestern Medicine study published in eLife.
There are currently no FDA-approved drugs that have been shown to effectively regenerate this coating, called myelin. This new strategy could represent a breakthrough in treating MS, according to Brian Popko, Ph.D., the William Frederick Windle Professor of Neurology and lead author of the study.
"This small molecule could be a potential therapeutic because not only have we shown that it provides protection to oligodendrocytes against inflammation, but we now are showing that it promotes myelin repair in an inflammatory environment," said Popko, who is also scientific director of the Division of Multiple Sclerosis and Neuroimmunology in the Ken and Ruth Davee Department of Neurology.
Oligodendrocytes are the cells that generate myelin, an insulating sheath that surrounds axons to allow rapid transportation of electrical impulses. Oligodendrocytes remain attached to myelin in order to properly maintain the myelin sheath, and a large population of oligodendrocyte progenitors—as much as 7 percent of all brain cells—lie in wait, ready to develop into fully-fledged oligodendrocytes when the original cells die.
In MS, oligodendrocytes are attacked by an autoimmune inflammatory response, and without their maintenance, the myelin sheath decays. If the lost myelin is not replaced in a timely manner, the axons degenerate, leading to profound neurological deficits.
Many possible therapeutics to repair myelin have been identified in preclinical studies, mostly focused on accelerating the rate at which progenitor cells are differentiated into full oligodendrocytes. However, none have been approved as MS therapeutics in humans.
Popko said one reason why these therapies have fallen short is the models in which they are tested; they lack the complex inflammatory environment present in the brain lesions of humans with MS.
"The inflammatory environment is far from ideal for the repair process; there are debris from previously present myelin, infiltrating lymphocytes and myriad proinflammatory cytokines not normally present in the brain," Popko said. "In purified cells and some demyelination mouse models, none of that is present."
A previous study from the Popko laboratory demonstrated that prolonging the integrated stress response can protect mature oligodendrocytes from inflammation, using more sophisticated mouse models that accurately mimic the inflammatory environment of the MS brain. However, the investigators were still curious about the effect on newly formed remyelinating cells.
In the current study, Popko, along with Yanan Chen, MD, Ph.D., a postdoctoral fellow in the Popko laboratory and lead author of the study, used both genetic and pharmacological methods to prolong the integrated stress response in inflammatory models of MS. The investigators discovered that the remyelinating cells were protected from harmful inflammation during the repair process.
"Prolonging the integrated stress response helps the cells withstand the adverse inflammatory environment and gives the cells more time to recover," Chen said.
Protecting both mature and remyelinating cells is a big step, one that Popko says makes this treatment method a promising discovery.
"Protection is better than having to repair, but either way you have to replace the oligodendrocytes that are lost," Popko said. "This therapy protects mature cells and the new cells that are necessary to replace cells that are lost."
Going forward, Popko and Chen will continue to investigate prolonging the integrated stress response in MS and are exploring opportunities for clinical trials, they said.
More information: Yanan Chen et al. Prolonging the integrated stress response enhances CNS remyelination in an inflammatory environment, eLife (2021). DOI: 10.7554/eLife.65469
Journal information: eLife
Provided by Northwestern University