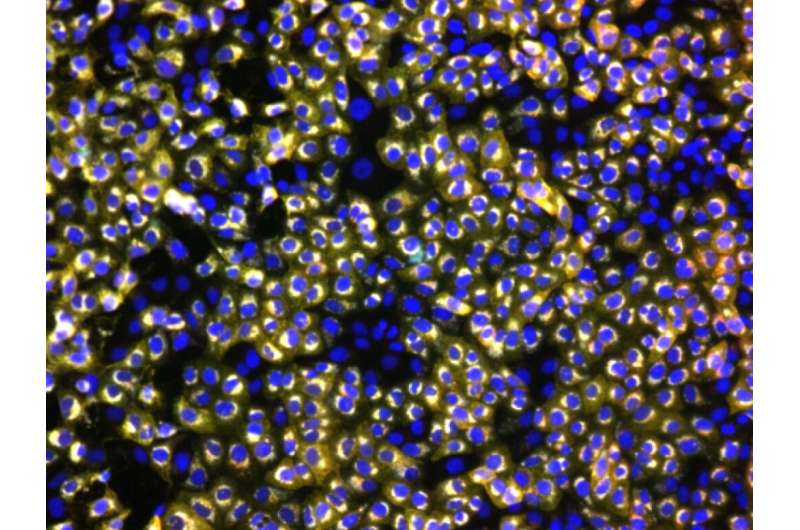
Representative microscopy image of SARS-CoV-2 infected cells stained with antibodies in convalescent plasma. Credit: Dr Michael Joyce, University of Alberta
Using convalescent plasma, Griffith University researchers have identified how it may be possible to make a future vaccine that will provide protection against all major strains of COVID-19.
Vaccines work by inducing antibodies that block the interaction between the receptor binding domain (RBD) of SARS-CoV-2's Spike protein and its receptor, angiotensin converting enzyme 2 (ACE2), which is present on human lungs.
To understand how antibodies block infection, the researchers from the Institute for Glycomics at Griffith University, with colleagues from the Gold Coast University Hospital, the University of Queensland, the University of Alberta, Canada, and Olymvax Pharmaceuticals, Chengdu, identified the minimal sequences of the RBD recognized by antibodies.
"We used plasma from convalescent patients (people who have recovered from COVID-19) and synthetic peptides to identify these minimal protein sequences,'' said lead author Professor Michael Good AO.
"We identified three small defined regions of the RBD that were recognized by antibodies from the immune convalescent individuals and then generated antibodies to each of these regions by vaccinating mice.
"We tested these sera individually and in combination for their ability to block the interaction of the RBD and ACE2 and observed that when sera were combined in pairs there was highly significant inhibition of binding of RBD to ACE2."
Co-lead author Dr. Manisha Pandey said their data indicated that, to be effective, antibodies must block more than one interacting region between RBD and ACE2.
"Of great interest, one of the pairs of epitopes did not contain any of the variant amino acids known to exist in the UK, South African or Brazilian variants.
"This means that it may be possible to use those specific small regions of the virus in a vaccine to induce antibodies capable of preventing infection with all main viral strains."
Professor Good said a successful vaccine must induce a durable response that will protect for at least one year enabling a COVID-19 vaccine to be administered in a similar way to vaccines for influenza.
"Vaccines must also have an excellent safety profile. This is particularly relevant for a vaccine against a disease for which the infection fatality rate in young people is very low. Vaccines that consist of only the essential regions of the virus required to induce protective antibodies will have such a safety profile.
"This research provides hope and new insight into how to make a vaccine that will provide protection against all major strains of the virus currently responsible for the pandemic."
Professor Mark von Itzstein AO, Director of the Institute for Glycomics was delighted with these research findings.
"Our Institute researchers have rapidly responded to tackle COVID-19 using a multi-prong approach and these latest results towards the development of next generation COVID-19 vaccines are very exciting. We hope that these vaccine candidates will deliver new solutions to this high-impact disease."
More information: Manisha Pandey et al. Antibodies to neutralizing epitopes synergistically block the interaction of the receptor‐binding domain of SARS‐CoV‐2 to ACE 2, Clinical & Translational Immunology (2021). DOI: 10.1002/cti2.1260
Provided by Griffith University