EU agency to issue more guidance on AstraZeneca's COVID shot
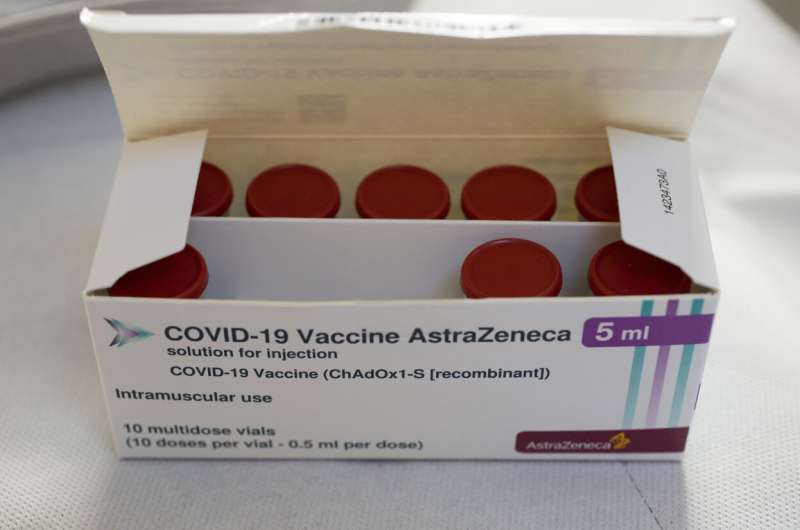
The European Medicines Agency is expected to provide updated guidance Friday on how countries across Europe should use the COVID-19 vaccine developed by AstraZeneca.
Earlier this month, the Amsterdam-based drug regulator for the 27-nation European Union said there was a "possible link" between the AstraZeneca vaccine and rare blood clotting disorders, but that the benefits of getting the shots outweighed the risks.
The agency's experts have since been considering related issues, including whether people who received a first AstraZeneca dose should be offered a second shot, and if there are specific risk factors that might make some people more vulnerable to developing the unusual blood clots.
The EMA previously described the clots as "very rare" side effects and said the vaccine labels should be modified so doctors and patients are aware of that.
It's still unclear exactly how frequently the rare blood clots occur. According to data from the U.K., which has administered more AZ vaccines than any other country, there were 30 such cases among 18 million inoculations, as of late March.
Last month, more than a dozen countries, mostly in Europe, suspended their use of the AstraZeneca jab over the blood clot issue. Most restarted—some with age restrictions—after the EMA said countries should continue using the vaccine.
The agency this week identified a similar possible link between blood clots and the COVID-19 vaccine developed by Johnson & Johnson. As with the AstraZeneca product, the EMA recommended labeling changes but said the benefits of getting vaccinated outweighed the risks.
Both the AstraZeneca and J&J vaccines are made using similar technology, and it's still unclear whether that might be partly responsible for the rare clotting disorders.
On Thursday, EU Health and Food Safety Commissioner Stella Kyriakides said she was expecting the EMA to issue guidance on whether people who received a first dose of the AstraZeneca vaccine should get a second dose, based on their sex or age.
To date, most of the rare clotting disorders have been reported in women aged under 60.
Although a research study began in the U.K. earlier this year to test whether it's safe and effective to mix and match different vaccines, including those made by AstraZeneca and Pfizer-BioNTech, no results are yet available.
Some scientists say it's too early to tell whether mixing vaccines is a wise strategy.
"We are at the limits of where science can give us reliable answers on that," said Stephen Evans, a vaccines expert at the London School of Hygiene and Tropical Medicine. He said the data published so far on the AstraZeneca vaccine prove it is helping to stop COVID-19 and that there is no evidence suggesting it shouldn't be used.
Any restrictions to limit the use of the AstraZeneca vaccine could be disastrous for the global immunization campaign. The U.N.-backed campaign known as COVAX, which is aiming to distribute coronavirus vaccines to poorer countries worldwide, is heavily dependent on the AstraZeneca shot.
© 2021 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.