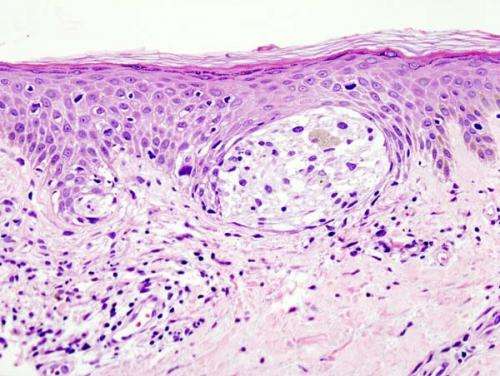
Melanoma in skin biopsy with H&E stain—this case may represent superficial spreading melanoma. Credit: Wikipedia/CC BY-SA 3.0
A dysfunctional immune system significantly contributes to the development of cancer. Several therapeutic strategies to activate the immune system to target cancer cells have been approved to treat different types of cancer, including melanoma. However, some patients do not show beneficial clinical responses to these novel and very promising immunotherapies. In a new article published in Proceedings of the National Academy of Sciences of the United States of America, Moffitt Cancer Center researchers demonstrate how an important defect in STING gene expression in melanoma cells contributes to their evasion from immune cell detection and destruction.
Several different mechanisms have been discovered that allow cancer cells to avoid immune cell detection and destruction, including defective T cell function, losses in expression of key proteins on tumor cells and defective cell signaling in both immune and tumor cells. An important signaling pathway that contributes to interactions between tumor cells and immune cells is the interferon signaling pathway. The interferon pathway increases expression of molecules that allow tumor cells to be recognized and killed by immune cells. One of the key molecules in the interferon signaling pathway is STING, which is activated by the protein cGAS.
Moffitt researchers previously demonstrated that STING activity is commonly suppressed and altered in a subset of melanomas, which prevents the ability of these tumor cells to be targeted by the immune system. The research team wanted to further the understanding of the importance of alterations in STING signaling in melanoma and determine how STING expression becomes suppressed. They focused on a process called epigenetic modification during which methylation groups are added to the DNA regulatory regions of genes, resulting in genes being turned off.
The researchers performed a series of laboratory experiments and discovered that the DNA regulatory region of the STING gene is highly modified by methylation groups resulting in loss of STING gene expression in certain melanoma cell lines. Importantly, they confirmed these findings in patient clinical samples of early and late-stage melanomas and showed similar methylation events and loss of expression of the upstream STING regulator cGAS.
Next, the researchers demonstrated that it is possible to reactivate expression of STING and/or cGAS with a demethylating drug or genetic approaches that overcome methylation. These interventions successfully turned on STING functional activity, resulting in increased interferon levels when triggered by STING agonist drugs that enabled the melanoma cells to now be recognized by immune cells and targeted for destruction.
These findings demonstrate for the first time that a strategy to overcome STING gene methylation can restore interferon signaling and immune cell activity in melanoma and improve a cell-based immunotherapy when combined with STING agonist drugs.
"These studies show the critical importance of an intact STING pathway in melanomas for optimal T cell immunotherapy success, and how to overcome a notable STING defect in melanoma cases of gene hypermethylation by a combination therapy," said James J. Mulé, Ph.D., senior author and associate center director for Translational Science at Moffitt. "Unless patients' melanomas are pre-screened for intact versus defective STING, it is not at all surprising that clinical trials of STING agonists have, to date, uniformly failed."
More information: Rana Falahat et al, Epigenetic reprogramming of tumor cell–intrinsic STING function sculpts antigenicity and T cell recognition of melanoma, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2013598118
Journal information: Proceedings of the National Academy of Sciences