Credit: Unsplash/CC0 Public Domain
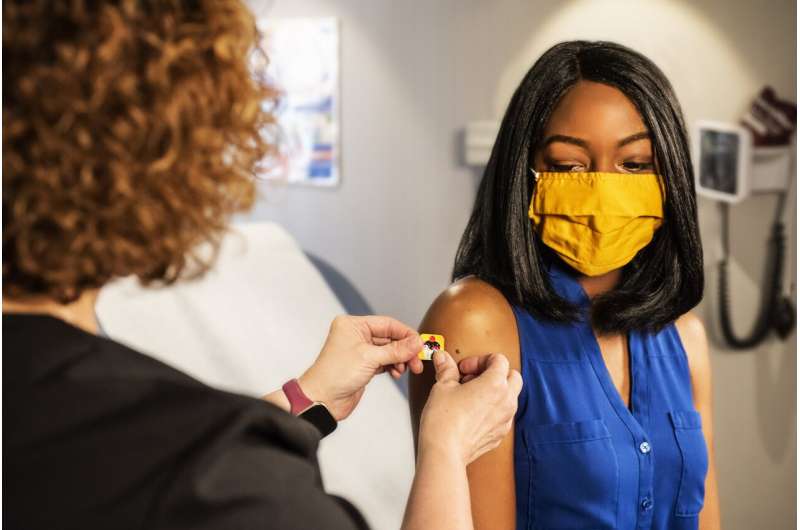
As a gastroenterology fellow treating patients with COVID-19, Keerthi Shah was eager to get the coronavirus vaccine. As a woman eight months pregnant with her first child, she was hesitant because there has been little research on how the vaccine affects pregnant people and their babies.
"Is something going to happen to the baby? I don't know," said Shah, 31, of Philadelphia. "The risk of me getting COVID and the side effects for the baby, that outweighs the risk of getting the COVID vaccine, in my mind."
Getting the coronavirus vaccine is a personal decision and one that is especially stressful for people like Shah who fall into categories among whom the vaccine was not tested in clinical trials.
The U.S. Centers for Disease Control and Prevention recently said pregnant people should get vaccinated, following a preliminary study that found "no obvious safety signals." Previously the agency had offered only vague guidance, suggesting that the vaccine was "unlikely to pose a specific risk for people who are pregnant" and recommending that patients talk to their obstetricians for help making a decision.
Philadelphia-area obstetricians said they are urging patients to make a decision based on facts, not fear, and to consider the risks of both getting the vaccine and going without it.
"My guidance as soon as the vaccine started rolling out has been if you have the opportunity to get it, get it," said Carlene Denis, an obstetrician with Tower Health and Axia Women's Health. "This gives you the best chance of being protected, and you are the vessel that carries this baby, so you give them the highest chance of being protected."
A year ago, Denis' office was bombarded with calls from pregnant patients wanting to know what risk the virus posed to their baby. Now, the calls are all about the risks of the vaccine.
That's troubling, she said.
Pregnant people do not seem more likely to get COVID-19, but are more vulnerable to severe illness if they do contract the virus.
Meanwhile what research is available about the vaccine during pregnancy suggests it is safe.
The largest study about the vaccine during pregnancy, published this week in the New England Journal of Medicine, offered reassuring findings: Rates of miscarriage, premature birth, and other complications among pregnant people who received the vaccine were comparable to those rates during pregnancy prior to the vaccine becoming available.
The preliminary results by CDC researchers are based on data from over 35,000 people who received the Moderna or Pfizer vaccine during pregnancy. The study was conducted before the Johnson & Johnson vaccine was available.
The report prompted the CDC to issue clear guidance recommending vaccination for all pregnant people for the first time since the vaccines became available.
"We know that this is a deeply personal decision," CDC director Rochelle Walensky said at a White House briefing on COVID-19 Friday, "and I encourage people to talk to their doctors and their primary care providers to determine what is best for them and for their baby."
The guidance is a shift from the vague and inconsistent advice that has come from top health organizations. The American College of Obstetricians and Gynecologists said the vaccine should not be withheld from pregnant people, while the World Health Organization has suggested pregnant people who are at high risk of exposure or vulnerable to severe illness because of other medical conditions should be vaccinated.
Other myths and misinformation—or just lack of information—have added confusion:
Could the vaccine affect fertility? Researchers have not found the vaccine to affect fertility and the CDC says there is no reason people who are trying to conceive should put off vaccination.
Will it interfere with the menstrual cycle? Some women have reported that their menstrual cycle was disrupted after getting the vaccine, though there has not been any significant research into this potential side effect. Denis said this likely has more to do with how the body responds to stress—in this instance the stress of developing a resistance to the coronavirus. The effect, which can happen following other vaccines, too, is temporary.
Nicole Lamborne, vice president of clinical operations for women's services at Virtua Health, said she expected the U.S. Food and Drug Administration's decision to pause use of the Johnson & Johnson vaccine following reports of a rare clotting disorder to make the decision even more difficult for her pregnant patients.
"The anxiety level among all our pregnant people has been extremely high throughout the pandemic, something like this put everyone on high alert," she said.
Prior to the Johnson & Johnson vaccine being paused, some of Lamborne's patients had canceled appointments when they found out they would be getting the one-dose shot because they were concerned about its safety during pregnancy, she said.
Some had religious objections to the vaccine, which was created using cloned fetal cells, while others were concerned about its similarities to the AstraZeneca vaccine that was suspended in Europe over a rare clotting complication.
Still others mistakenly believed it contained live virus, a type of vaccine that is not recommended during pregnancy.
Unlike the Pfizer and Moderna vaccines, which are made with mRNA, the Johnson & Johnson vaccine uses a viral vector, which is a different virus used to teach the body to react to the coronavirus. This type of vaccine will not give people COVID-19 or the virus used as the "vector" and have been safely used among pregnant people in other vaccines.
Surprisingly, she said more women have been signing up since the FDA paused the Johnson & Johnson vaccine, because they know they will receive either the Pfizer or Moderna shots.
"Which risk do you take? Do you risk getting the disease, or risk taking the vaccine? That's what it comes down to for most patients," Lamborne said.
Lamborne said she is encouraging "almost all" of her patients to get the vaccine, rather than risk getting sick.
Most of Denis' patients have made up their minds about whether to get vaccinated—the more difficult decision is about when in their pregnancy to get it, she said.
She typically recommends waiting until after the first trimester, which is the most critical period for a fetus' development. Some are waiting until after they give birth, but many people are still afraid of being in the hospital and want to be vaccinated before their stay, she said. Plus, research suggests that immunity is transferred in utero and through breast milk, so vaccine during pregnancy or shortly after could protect the baby.
Shah scheduled her vaccine for January, several weeks before her due date. But baby Lily arrived a month early, a few days before Shah got her first dose of the vaccine.
Three months later, Lily is doing well and Shah has no regrets about her decision. She's even applied to be part of a breast milk study among vaccinated people.
"COVID is such a big part of our lives—everything has changed so much," she said. "I think the safest way to come out of this is for everyone to get vaccinated."
Journal information: New England Journal of Medicine
©2021 The Philadelphia Inquirer, LLC.
Distributed by Tribune Content Agency, LLC.