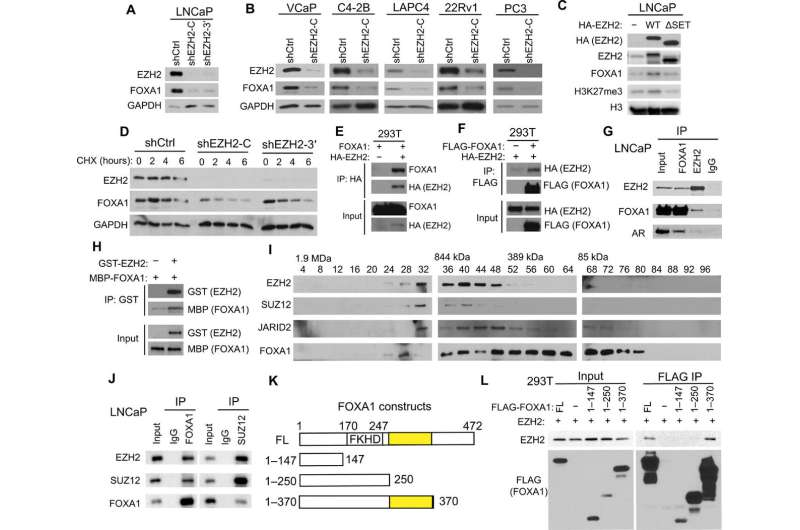
Fig. 1 EZH2 increases FOXA1 protein stability through physical interaction. (A and B) PCa cells were infected with control (shCtrl) or two independent shEZH2 lentiviruses (shEZH2-C and shEZH2-3′) for 72 hours and subjected to WB. (C) LNCaP cells were infected with the indicated EZH2 lentivirus for 96 hours and analyzed by WB. (D) LNCaP cells were treated with protein synthesis inhibitor CHX (150 μg/ml) for 0, 2, 4, and 6 hours before they were collected for WB. (E and F) 293T cells were transfected with indicated plasmids for 48 hours. Coimmunoprecipitation (co-IP) was performed using a hemagglutinin (HA) (E) or FLAG antibody (F), followed by WB. (G) Co-IP with FOXA1, EZH2, or immunoglobulin G (IgG) antibody in LNCaP nuclear lysates followed by WB. (H) Purified recombinant GST–green fluorescent protein (GFP) or GST-EZH2 coupled to GST beads were used to pull-down purified MBP-FOXA1, which was analyzed by WB using an MBP antibody. (I) Nuclear extracts from LNCaP cells were fractionated and subjected to WB. (J) Co-IP using LNCaP nuclear lysates was performed with FOXA1, SUZ12, or IgG antibody. (K and L) EZH2 was cotransfected into 293T cells along with various FLAG-tagged FOXA1 domain constructs (K). Co-IP assay was performed with anti-FLAG M2 beads followed by WB using anti-EZH2 (L). EZH2-binding domains are highlighted in yellow. Credit: Science Advances (2021). DOI: 10.1126/sciadv.abe2261
Northwestern Medicine scientists have discovered a mechanism that makes a prostate cancer-causing protein called FOXA1 more resilient, according to a study published in Science Advances.
The findings shed light on why some prostate cancers don't respond to certain drugs and chart a path to a possible future therapy, according to Jindan Yu, MD, Ph.D., professor of Medicine in the Division of Hematology and Oncology and senior author of the study.
"This shows how FOXA1 is stabilized so it can promote cancer growth," said Yu, who is also a professor of Biochemistry and Molecular Genetics and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
FOXA1 is a key regulator of the cell cycle, promoting cell growth and proliferation. In some prostate cancers, FOXA1 is upregulated and fuels cancer growth, but the specific pathways that cause its upregulation were unknown, according to Yu.
Using a broad array of biochemistry assays, the investigators discovered that FOXA1 is a primary non-histone target of EZH2, a regulatory enzyme known to methylate histones to promote prostate cancer growth. Specifically, EZH2 methylates FOXA1, which helps remove an ubiquitin tail on FOXA1, eliminating one of the most common methods by which proteins are degraded.
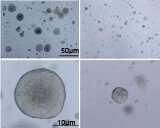
Patient-derived prostate cancer cells (left) with high FOXA1 grow large, but the dual-inhibitor therapy reduced their growth considerably (right). Credit: Northwestern University
"Normally, this ubiquitin tail is attached to the protein which leads it to the proteasome to be degraded," Yu said. "This little tail is removed so it is more stable in the cells."
EZH2 inhibitors have shown limited efficacy in prostate cancer cells. In FOXA1-driven cancers, however, Yu said she believes this inhibitor could be more effective. Further, combining this inhibitor with a de-ubiquitinase inhibitor that limits EZH2's ability to stabilize FOXA1 produced even better results in human and mouse models of prostate cancer.
"We think this drug combination has a lot of potential, especially in cancers with high FOXA1," Yu said.
More information: Su H. Park et al. Posttranslational regulation of FOXA1 by Polycomb and BUB3/USP7 deubiquitin complex in prostate cancer, Science Advances (2021). DOI: 10.1126/sciadv.abe2261
Journal information: Science Advances
Provided by Northwestern University