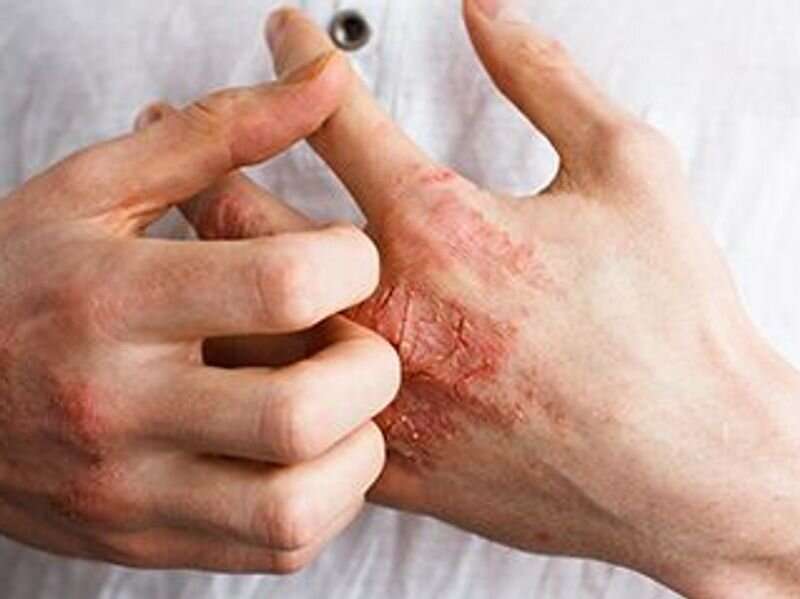
Baricitinib demonstrates sustained long-term efficacy in patients with moderate-to-severe atopic dermatitis, according to a study published online May 12 in JAMA Dermatology.
Jonathan I. Silverberg, M.D., from the George Washington University School of Medicine in Washington, D.C., and colleagues evaluated the long-term (68 weeks) efficacy of baricitinib in adults with moderate-to-severe atopic dermatitis who were treatment responders or partial responders in phase 3 monotherapy studies.
The researchers found that of the responder/partial responder population treated with 4 mg baricitinib (70 patients; mean age, 36.7 years; 60 percent men), 45.7 percent achieved validated Investigator Global Assessment for Atopic Dermatitis scores of 0 or 1 (vIGA-AD [0,1]) at week 16; the proportion was 47.1 percent at week 68. Seventy percent of participants achieved improvement of ≥75 percent in the Eczema Area and Severity Index (EASI75) at week 16 versus 55.7 percent at week 68. Among the responder/partial responder population treated with 2 mg baricitinib (54 patients; mean age, 32.8 years; 51.9 percent men), 46.3 percent achieved vIGA-AD (0,1) at week 16 versus 59.3 percent at week 68. At week 16, improvement in the EASI75 score was 74.1 percent compared with 81.5 percent at week 68.
"Overall, these findings provide support that baricitinib may be a longer-term treatment option for patients with moderate-to-severe atopic dermatitis," the authors write.
Several authors disclosed financial ties to pharmaceutical companies, including Eli Lilly and Company, which manufactures baricitinib and funded the study.
More information: Abstract/Full Text
Journal information: JAMA Dermatology
Copyright © 2021 HealthDay. All rights reserved.