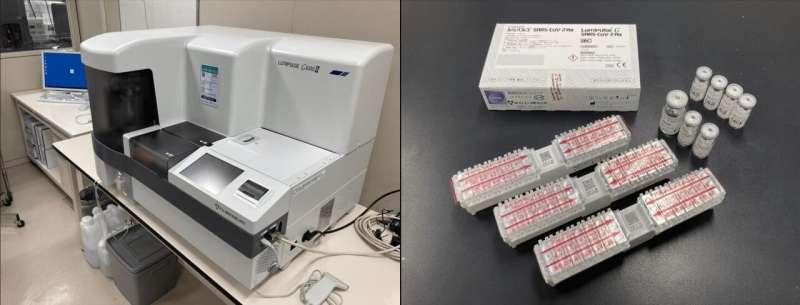
The Lumipulse G600II instrument (left) and the Lumipulse SARS-CoV-2 Ag kit (right), both manufactured by Fujirebio, which were used in this study for the quantification of SARS-CoV-2 in saliva samples. Credit: Shinichi Fujisawa
Scientists from Hokkaido University have shown that an antigen-based test for quantifying SARS-CoV-2 in saliva samples is simple, rapid, and more conducive for mass-screening.
More than a year into the COVID-19 pandemic, the RT-PCR test remains the gold standard for detection of the SARS-CoV-2 virus. This method requires trained personnel at every step, from collection of nasopharyngeal swab (NPS) samples to interpretation of the results; in addition, the entire process ranges from 24–48 hours on average. As the virus can be transmitted by an infected person before symptoms develop, and is even transmitted by individuals who are asymptomatic, the ability to screen a large number of people quickly is vital to controlling and preventing the spread of the pandemic. Faster methods to detect the SARS-CoV-2 antigens have been developed, but they are not as sensitive as the RT-PCR test. In June 2020, a novel antigen-based kit, Lumipulse SARS-CoV-2 Ag kit (Lumipulse), was developed by Fujirebio to quantitatively measure the viral antigen in biological samples within 35 minutes.
A team of scientists from Hokkaido University have used the antigen kit to detect SARS-CoV-2 in saliva samples, and have assessed the efficiency and accuracy of the test compared to RT-PCR. Their findings show that the antigen detection kit, which is used to perform chemiluminescent enzyme immunoassay (CLEIA), can rapidly detect SARS-CoV-2 with good accuracy in these samples. The study was published in the journal The Lancet Microbe.
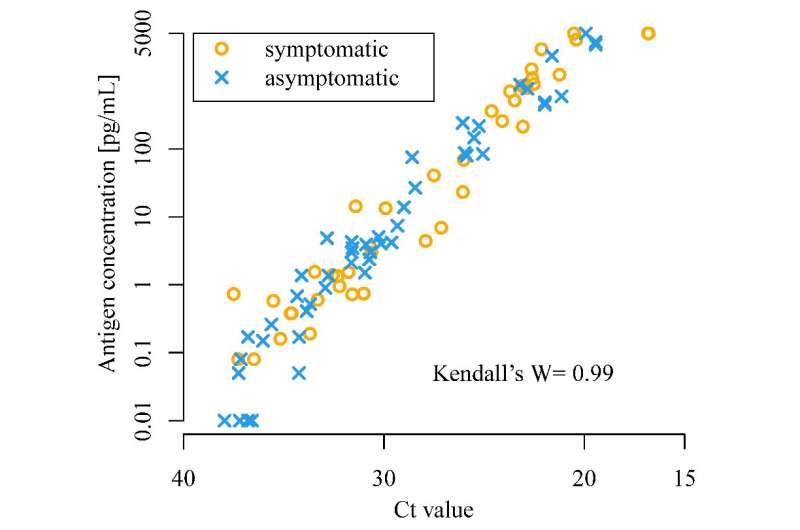
The CLEIA test (y-axis) for SARS-CoV-2 correlates well with the RT-PCR test (x-axis). Orange circles indicate symptomatic cases and blue crosses indicate asymptomatic cases. Credit: Isao Yokota, et al. The Lancet Microbe. May 19, 2021
The scientists tested 2056 individuals from three cohorts: patients with clinically confirmed COVID-19, individuals who had contacted patients with COVID-19, and individuals tested on arrival at Tokyo and Kansai International Airports. Saliva samples were collected from all individuals and used for RT-PCR tests as well as CLEIA using Lumipulse. The results of both were compared to determine the usefulness of CLEIA.
The scientists found that CLEIA is a reliable test, as it correlates well with RT-PCR. CLEIA alone can be used to detect SARS-CoV-2 within an hour; however, using CLEIA for screening and RT-PCR for confirmation increases the accuracy of diagnosis.
The benefit of using saliva samples is the ease of collection: it is quick and can be collected by the individuals being tested, reducing the risk that healthcare workers are exposed to the virus. Furthermore, self-collection of saliva allows multiple samples to be collected simultaneously for expeditious screening of visitors at large gatherings.
Combined CLEIA and RT-PCR testing on saliva samples has already been implemented at Japanese airport quarantines, and the authors recommend adopting it at a wider scale to rapidly screen for SARS-CoV-2.
More information: Isao Yokota et al, A novel strategy for SARS-CoV-2 mass screening with quantitative antigen testing of saliva: a diagnostic accuracy study, The Lancet Microbe (2021). DOI: 10.1016/S2666-5247(21)00092-6
Provided by Hokkaido University