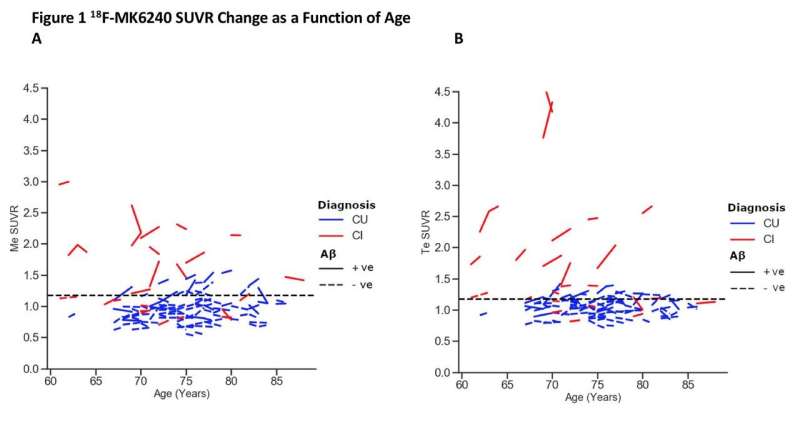
Tau accumulation over one year measured in composite A) mesial temporal ROI; and B) temporoparietal ROI in cognitively unimpaired participants (blue) and cognitively impaired participants (red). The CI group included participants with clinical mild cognitive impairment and dementia. Higher rates of tau accumulation were observed in participants on the AD continuum (CU Aβ+ve and CI Aβ+ve). Participants with the highest baseline tau and rates of tau accumulation were younger and more likely to be CI Aβ+ve. Credit: N Krishnadas et al. Florey Department of Neurosciences & Mental Health, The University of Melbourne; Department of Molecular Imaging & Therapy, Austin Hospital, Melbourne.
A novel positron emission tomography (PET) radiotracer has been shown to effectively measure increases in brain tau—a distinguishing characteristic of Alzheimer's disease—before any symptoms of the disease are observed. With the potential to measure increases in tau over a long period of time, this tracer offers an important tool to assess the effectiveness of Alzheimer's disease treatments in clinical trials. This research was presented at the Society of Nuclear Medicine and Molecular Imaging 2021 Annual Meeting.
Tau is a protein commonly found in healthy brain neurons. In people with certain brain disorders, like Alzheimer's disease, chemical changes cause tau proteins to accumulate in various parts of the brain. As such, tau is valuable as a biomarker for measuring disease progression.
In the study, researchers sought to detect patterns and rates of tau accumulation in both a cognitively normal aging population and in those with Alzheimer's disease. PET imaging with the novel radiotracer 18F-MK6240 was performed on all participants at baseline and after 12 months. After each scan, uptake of the radiotracer was measured in multiple areas of the brain.
Increases in tau were measured in both participant groups and longitudinal tau imaging was effective in discriminating between the two cohorts. The uptake of 18F-MK6240 was higher at baseline and after one year in participants who were on the Alzheimer's disease continuum in comparison to the cognitively normal aging participants.
"The effectiveness of the 18F-MK6240 tracer is important for drug trials that aim to measure whether or not treatments to remove tau from the brain are actually working," said Christopher Rowe, BMBS, FRACP, MD, FAANMS, director of molecular imaging research at Austin Health and director of the Australian Dementia Network in Melbourne, Australia. "Use of the radiotracer will allow researchers to select people at different stages of Alzheimer's disease for clinical trials, which ultimately may speed the development of effective treatments for the disease."
More information: Abstract 105. "18F-MK6240 longitudinal tau PET in ageing and Alzheimer's disease," Natasha Krishnadas, Florey Department of Neurosciences & Mental Health, The University of Melbourne, Parkville, Victoria, Australia, and Department of Molecular Imaging & Therapy, Austin Hospital, Heidelberg, Victoria, Australia; Vincent Dore, CSIRO Biomedical Imaging Health & Biosecurity Flagship, Parkville, Victoria, Australia, and Department of Molecular Imaging & Therapy, Austin Hospital, Heidelberg, Victoria, Australia; Rachel Mulligan, Regan Tyrrell, Svetlana Bozinovski, Kun Huang, Fiona Lamb and Victor Villemagne, Department of Molecular Imaging & Therapy, Austin Hospital, Heidelberg, Victoria, Australia; Samantha Burnham, CSIRO Biomedical Imaging Health & Biosecurity Flagship, Parkville, Victoria, Australia; and Christopher Rowe, Department of Molecular Imaging & Therapy, Austin Hospital, Heidelberg, Victoria, Australia, Australian Dementia Network (ADNeT), Victoria, Australia, and Florey Department of Neurosciences & Mental Health, The University of Melbourne, Parkville, Victoria, Australia.
Provided by Society of Nuclear Medicine and Molecular Imaging