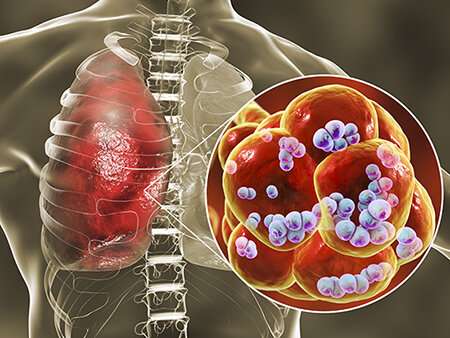
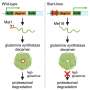
This novel virulence trait, which increases severity of S. pneumoniae superinfection, involves pneumococcal surface protein A, now identified as an adhesin. Credit: University of Alabama at Birmingham
A bout with flu virus can be hard, but when Streptococcus pneumonia enters the mix, it can turn deadly.
Now researchers have found a further reason for the severity of this dual infection by identifying a new virulence mechanism for a surface protein on the pneumonia-causing bacteria S. pneumoniae. This insight comes more than three decades after discovery of that surface protein, called pneumococcal surface protein A, or PspA.
This new mechanism had been missed in the past because it facilitates bacterial adherence only to dead or dying lung epithelial cells, not to living cells. Heretofore, researchers typically used healthy lung cell monolayers to search for bacterial adhesins that aid infection. Virus killing of lung cells during flu was found to set the stage for S. pneumonia attachment to the airway, thereby worsening disease and pneumonia.
The research, published in the journal Cell Reports, was led by Carlos Orihuela, Ph.D., and David Briles, Ph.D., professor and professor emeritus in the University of Alabama at Birmingham Department of Microbiology. Orihuela and Briles say their findings provide further explanation for how an infection by influenza A flu virus—followed by S. pneumoniae superinfection—causes severe pneumonia and a high death rate. The mechanism also points to possible improvements for disease treatment and vaccination.
A historical example of the deadly synergy of flu infection followed by S. pneumoniae superinfection is found in banked lung samples from the 1918 Spanish influenza pandemic that killed 40 million to 50 million people—the vast majority of these samples showed co-infection or secondary infection with S. pneumonia.
The UAB research on PspA began with some head-scratching results from experimental lung infections of mice with influenza A, followed by either wild-type S. pneumonia that has the intact PspA gene, or a mutant S. pneumoniae that lacks PspA. Lung homogenates from mice infected with the wild-type had much higher numbers of S. pneumonia bacteria than lungs infected with the mutant. However, when researchers washed the interiors of the lungs and collected that bronchoalveolar lavage fluid, they counted similar numbers of the wild-type S. pneumonia and the mutant.
"This unexpected result was interpreted to mean that wild-type S. pneumoniae were more resistant to dislodgement than S. pneumonia with a pspA gene deletion, and it served as rationale for further experimentation," Orihuela said.
From this clue, the researchers were able to show that PspA functions as an adhesin to dying host cells, in addition to its several other previously established virulence mechanisms. The researchers also detailed the molecular mechanism of this bacterial adherence.
Both influenza A infection and release of the S. pneumoniae toxin pneumolysin cause death of lung epithelial cells. As they are dying, cells' phosphatidylserine residues get flipped to the outer cell membrane, where they bind the host enzyme glyceraldehyde-3-phosphate dehydrogenase, or GAPDH. In turn, the S. pneumoniae PspA on the surface of the bacteria binds to the GAPDH. PspA-GAPDH-mediated binding to lung cells increased S. pneumoniae localization in the lower airway, and this was enhanced by pneumolysin exposure or co-infection with influenza A virus.
Tests with fragments of the PspA protein showed that a 52-amino acid portion of the protein—from amino acid 230 to 281—was required for GAPDH binding. Instilling one of those binding fragments into the lungs of influenza-infected mice reduced the disease severity of S. pneumoniae superinfection, presumably through binding competition.
"Our findings support the targeting of regions of PspA for therapeutic and vaccine development against influenza A/Streptococcus pneumoniae superinfections," Orihuela said. "Importantly, and despite more than 30 years since its discovery, PspA was not previously shown to function as an adhesin. Thus, our finding of PspA's role in adherence substantially advances our knowledge on the interactions of S. pneumoniae with its host."
Besides Orihuela and Briles as co-senior authors, Sang-Sang Park and Norberto Gonzalez-Juarbe are co-first authors of the study, "Streptococcus pneumoniae binds to host GAPDH on dying lung epithelial cells worsening secondary infection following influenza." The four researchers hold a provisional patent on PspA.
More information: Sang-Sang Park et al, Streptococcus pneumoniae binds to host GAPDH on dying lung epithelial cells worsening secondary infection following influenza, Cell Reports (2021). DOI: 10.1016/j.celrep.2021.109267
Journal information: Cell Reports
Provided by University of Alabama at Birmingham