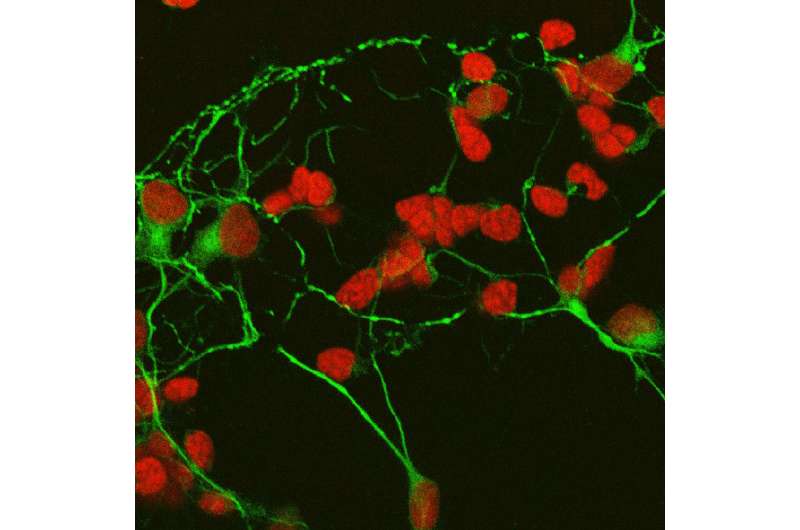
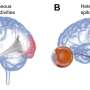
Amacrine and photoreceptor cells treated with PEDF in culture. PEDF stimulated extensive amacrine axonal outgrowth and protected photoreceptors from cell death, simultaneously promoting their differentiation. Cell nuclei are labeled in red, and cellular skeleton in green. Credit: Patricia Becerra, NEI
Researchers at the National Eye Institute (NEI) have determined how certain short protein fragments, called peptides, can protect neuronal cells found in the light-sensing retina layer at the back of the eye. The peptides might someday be used to treat degenerative retinal diseases, such as age-related macular degeneration (AMD). The study published today in the Journal of Neurochemistry.
A team led by Patricia Becerra, Ph.D., chief of the NEI Section on Protein Structure and Function, had previously derived these peptides from a protein called pigment epithelium-derived factor (PEDF), which is produced by retinal pigment epithelial cells that line the back of the eye.
"In the eye, PEDF protects neurons from dying. It prevents the invasion of blood vessels, it prevents inflammation, it has antioxidant properties—all these are beneficial properties," said Becerra, the senior author of the study. Her studies suggest that PEDF is part of the eye's natural mechanism for maintaining eye health. "PEDF may have a role for treating eye disease. If we want to exploit the protein for therapeutics, we need to separate out the regions responsible for its various properties and determine how each of them works."
The team used a well-known cell culture model system where immature retinal cells are isolated from the eyes of newborn rats and grown in a dish with minimal nutrients. The system includes not only the retina's light-sensing photoreceptors, but additional types of neurons that help the retina process and transmit visual information to the brain.
"Our model system—using cells isolated from the animal—lets us tease out the individual processes and mechanisms behind PEDF's protective effects," said Germán Michelis, graduate student and the study's first author.
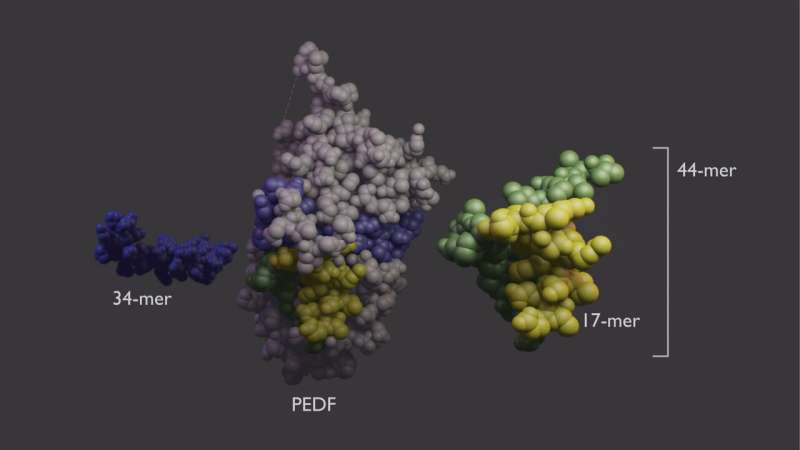
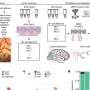
The PEDF protein has functionally distinct domains. The Becerra lab previously found that each domain can work independently. One area, which is called the 34-mer because it is formed by 34 amino acid building blocks, halts blood vessel growth. Aberrant blood vessel growth is central to retinal diseases such as AMD and diabetic retinopathy. The second PEDF domain, called the 44-mer, provides anti-death signals to retinal neurons. The 44-mer can also stimulate neurons to grow neurites, finger-like projections that help the neurons communicate with their neighbors. A shorter version of the 44-mer of only 17 amino acids (17-mer) has identical activities.
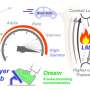
PEDF protein (center) has two domains with different functions. The 34-mer (blue, left) has anti-angiogenic properties. The 44-mer (green and yellow, right) protects and stimulates neurons. The 17-mer (yellow) is a smaller region of the 44-mer with the same function. Credit: Lesley Earl, NEI
Michelis and colleagues tested whether the 44-mer could protect immature retinal cells in a dish. Without the presence of proteins and other cells in their usual retinal environment, immature photoreceptors quickly die but can be preserved with PEDF.
They found that both the 44-mer and 17-mer were as capable of saving these photoreceptors as full-length PEDF.
The researchers also found that PEDF activity appears to be most needed at a specific point in photoreceptor cell development. Light detection takes place in a part of the photoreceptor known as the outer segment, where light-sensing opsin proteins are concentrated. The scientists found that when a photoreceptor cell is just beginning to create its outer segments, PEDF triggers the movement of opsin into the budding outer segment, where it belongs.
Along with photoreceptors, the retina is packed with several other types of neurons, which work together to process visual signals. Via neurites, amacrine neurons form connections, called synapses, to the cells that forward these visual signals to the brain. Becerra and colleagues found that PEDF stimulates amacrine cells to develop neurites in their cell culture model and that the 44-mer and 17-mer were at least as effective—or better—at stimulating these connections than the native protein.
Further, the 44-mer and 17-mer peptides work by binding to a protein receptor (PEDF-R) on the surface of neurons. PEDF activates PEDF-R, which processes molecules like docosahexaenoic acid (DHA), an omega-3 fatty acid critical for babies' development and for eye health. PEDF-R was discovered previously by the Becerra lab.
"We've known for a long time that DHA is important for retinal health. We think PEDF signaling might be a key component of regulating omega-3 fatty acids like DHA, both during eye development and in maintaining the eye's health over time," said Becerra. "We're hoping that we can harness some of these protective effects in a peptide-based therapeutic approach in the near future."
More information: Germán Michelis et al, Pigment Epithelium‐derived Factor (PEDF) and Derived Peptides Promote Survival and Differentiation of Photoreceptors and Induce Neurite‐outgrowth in Amacrine Neurons, Journal of Neurochemistry (2021). DOI: 10.1111/jnc.15454
Journal information: Journal of Neurochemistry
Provided by National Eye Institute