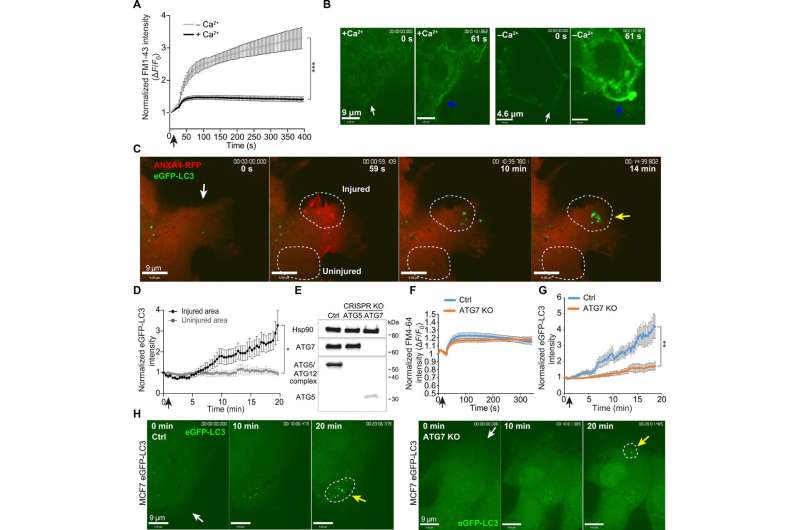
Fig. 1 Laser injury leads to ATG7-dependent formation of LC3-positive vesicles around the plasma membrane repair area. (A) MCF7 cells were injured by ablation laser in medium with or without Ca2+. Mean ± SEM of normalized FM1-43 cytoplasmic dye levels, 11 cells per condition from three experiments, P < 0.0001 (AUC analysis, unpaired t test with Welsh’s correction). Black arrow, injury time point. (B) Representative images for FM1-43 intensity of MCF7 cells injured in Ca2+-containing medium (left) and Ca2+-deficient medium (right) before and after injury (61 s). White arrows, injury site. Blue arrows point to FM1-43 dye accumulation. (C) Representative sequential images of ANXA4-tRFP–transfected MCF7 eGFP-LC3 cells exposed to laser injury. Yellow arrow, LC3 puncta formation. White circles, eGFP-LC3 quantification. Also see movie S1. (D) eGFP-LC3 intensity after laser injury in injured areas compared to control. Fifteen individual cells from three experiments (mean ± SEM, paired t test of AUC values). Black arrow, injury time point. (E) Immunoblot for ATG7, ATG5, and the ATG5/ATG12 complex (Hsp90, loading control) of lysates from MCF7 eGFP-LC3 CRISPR KO cells: nontargeted control, ATG5 CRISPR KO, and ATG7 CRISPR KO. (F) Cell membrane repair kinetic upon laser injury in ATG7 CRISPR KO and Ctrl cells as measured by FM4-64 cytoplasmic dye levels from 11 cells per cell line (from three experiments). Mean ± SEM. Black arrow, injury time point. (G) Analysis of eGFP-LC3 intensity at the injury site in MCF7 ATG7 CRISPR KO and Ctrl cells (mean ± SEM, 16 cells from three experiments per cell line, unpaired t test of AUC values, Welsh’s correction). (H) Representative sequential images of eGFP-LC3 puncta formation in Ctrl (left) and in ATG7 CRISPR KO cells (right) after laser injury. White arrows indicate injury sites, yellow arrows indicate area of LC3 puncta formation, and white circles indicate the area used for eGFP-LC3 quantification. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Credit: DOI: 10.1126/sciadv.abg1969
To survive life-threatening injuries, cancer cells use a technique in which they eat parts of the membrane surrounding them. This is shown for the first time in research from a team of Danish researchers.
It is the membrane of cancer cells that is at the focus of the new research now showing a completely new way in which cancer cells can repair the damage that can otherwise kill them.
In both normal cells and cancer cells, the cell membrane acts as the skin of the cells. And damage to the membrane can be life threatening. The interior of cells is fluid, and if a hole is made in the membrane, the cell simply floats out and dies—a bit like a hole in a water balloon.
Therefore, damage to the cell membrane must be repaired quickly, and now research from a team of Danish researchers shows that cancer cells use a technique called macropinocytosis. The technique, which is already a known tool for cells in other contexts, consists in the cancer cells pulling the intact cell membrane in over the damaged area and sealing the hole in a matter of minutes. Next, the damaged part of the cell membrane is separated into small spheres and transported to the cells' lysosomes, where they are broken down.
In the laboratory, the researchers damaged the membrane of the cancer cells using a laser that shoots small holes in the membrane and triggers macropinocytosis. Here, they can see that if the process is inhibited with substances blocking the formation of the small membrane spheres, the cancer cell can no longer repair the damage and dies.
"Our research provides very basic knowledge about how cancer cells survive. In our experiments, we have also shown that cancer cells die if the process is inhibited, and this points towards macropinocytosis as a target for future treatment. It is a long-term perspective, but it is interesting," says group leader Jesper Nylandsted from the Danish Cancer Society's Research Center and the University of Copenhagen, who has headed the new research and who for many years has investigated how cancer cells repair their membranes.
Possibility of recycling
One of the most dangerous properties of cancer is when the disease spreads in the body. If tumors occur in new parts of the body, the disease becomes more difficult to treat and typically requires more extensive forms of treatment. It is also when cancer cells spread through the body's tissues that they are particularly prone to damage to their membrane.
Researchers at the Danish Cancer Society have previously shown how cancer cells can use another technique to repair the membrane, namely by tying off the damaged part, rather like when a lizard throws its tail.
However, the experiments in the laboratory could indicate that especially the aggressive cancer cells use macropinocytosis. This may be due to the fact that the cancer cell has the opportunity to reuse the damaged membrane when it is degraded in the lysosomes. This type of recycling will be useful for cancer cells because they divide frequently, requiring large amounts of energy and material for the new cells.
And although the researchers have now published the new results, their work is not over. This is explained by another member of the research team, postdoc Stine Lauritzen Sønder: "We continue to work and investigate how cancer cells protect their membranes. In connection with macropinocytosis in particular, it is also interesting to see what happens after the membrane is closed. We believe that the first patching is a bit rough and that a more thorough repair of the membrane is needed afterwards. It can be another weak point in the cancer cells, and is something we want to examine closer," she says.
More information: Stine Lauritzen Sønder et al, Restructuring of the plasma membrane upon damage by LC3-associated macropinocytosis, Science Advances (2021). DOI: 10.1126/sciadv.abg1969
Journal information: Science Advances
Provided by University of Copenhagen