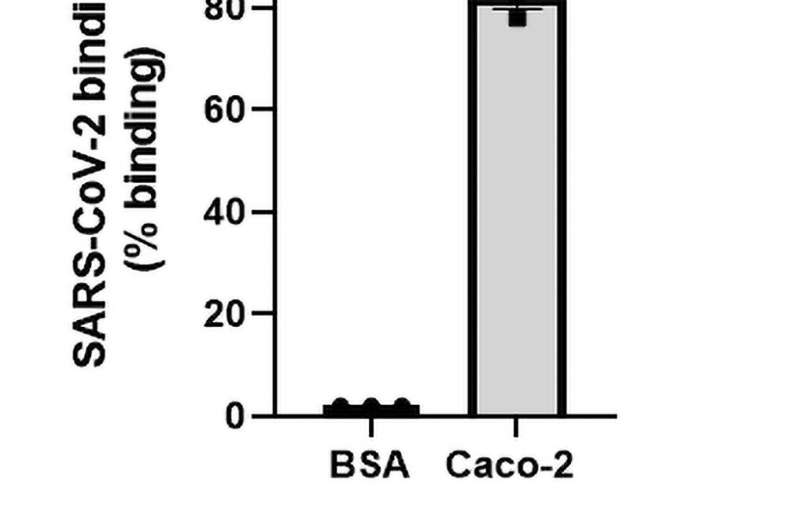
Fig 1. SARS-CoV-2 binds to human epithelial cells and causes permeability. (A) SARS-CoV-2 (1.08 x 105 TCID50/mL) was added to either a control surface (BSA) or human epithelial cells (Caco 2). Cells were allowed to adhere to immobilised SARS-CoV-2 and lysed with pNPP, a fluorescent substrate against alkaline phosphatase expressed within cells. The fluorescent signal emitted by pNPP correlated to the number of cells adhered and was read at 405 nm. Epithelial cells significantly interacted with SARS-CoV-2 (P<0.001; paired t-test, N = 3). (B) Caco-2 was seeded onto transwell inserts and infected with SARS-CoV-2 at MOI = 0.4. Permeability was measured using Fluorescein isothiocyanate-dextran (FITC-Dextran, 40kDa) across 0 hours, 24 hours, and 48 hours. FITC-Dextran passes through the epithelial cells into the lower chamber and is proportionate to their permeability. The extent of permeability was measured by quantifying the fluorescent levels of FITC-Dextran at 490/520 nm. Cell permeability significantly increased throughout the time course (P<0.01; P<0.0001; ANOVA f-value = 46.84, N = 3).
Scientists have identified a drug that can prevent the virus that causes COVID-19 from binding to human cells, potentially preventing damage to the lung, clot formation and the development of sepsis.
The study, led by researchers from RCSI University of Medicine and Health Sciences, is published in PLOS ONE.
The researchers identified that a mutation, present in all the variants of the virus to date, creates an additional binding site in the virus' spike protein. This additional binding site increases viral impact in the body, including damage to the lung tissue that causes breathing problems in COVID-19 patients.
The significant damage to the lung tissue allows the virus to spread from the lungs to the bloodstream, where it can cause clots and vascular damage.
In pre-clinical testing, a drug called cilengitide successfully prevented the virus from causing the tissue damage associated with COVID-19 by stopping the virus from sticking to the cell types that line the lungs and blood vessels.
"More pre-clinical and clinical testing is needed before this treatment can be used on patients, but the results are very promising," said Professor Steve Kerrigan, the study's senior author and Deputy Head of the RCSI School of Pharmacy and Biomolecular Sciences.
"It is imperative that we continue to develop treatments for COVID-19 for the many people who will not have access to vaccines and for patients with breakthrough infections. Our research in the lab has shown that cilengitide has the potential to significantly reduce the deaths associated with COVID-19."
More information: Danielle Nader et al, SARS-CoV-2 uses major endothelial integrin αvβ3 to cause vascular dysregulation in-vitro during COVID-19, PLOS ONE (2021). DOI: 10.1371/journal.pone.0253347
Journal information: PLoS ONE
Provided by Royal College of Surgeons in Ireland (RCSI)