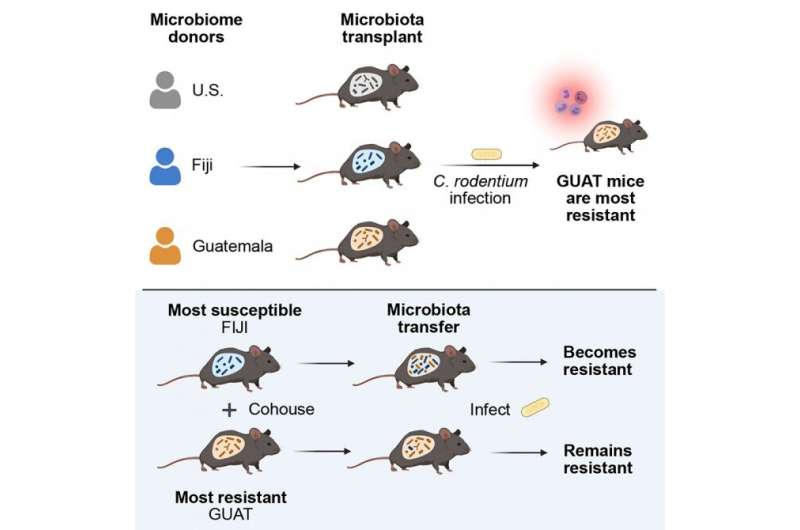
Cornell researchers “humanized” mice with microbiota from three global populations, then exposed them to Citrobacter rodentium, a model for severe intestinal infections like E. coli, to see how they responded. Credit: Cell Reports
The gut microbiome is a diverse environment, jam-packed with up to 1,000 different species of bacteria. Human populations around the globe have significant differences in the composition of their gut microbiomes, which can impact their health in unique ways that have not been completely understood.
Complicating matters, gut microbiome research has predominantly focused on health subjects important to high-income countries like the United States and in Europe, leaving out much of the world's health problems.
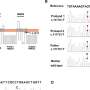
A Cornell-led project has sought to fill in some of these gaps by studying mice that were "humanized" with microbiota from three global populations – in the U.S., Fiji and Guatemala – and their resistance to severe intestinal infection. The researchers found that microbial differences alone can impact immune responses, and quite quickly at that. They also observed that housing the mice together so that they shared microbiota helped mice with low resistance to infection become more resilient.
The group's paper, "Geographic Differences in Gut Microbiota Composition Impact Susceptibility to Enteric Infection," published July 27 in Cell Reports. The paper's lead author is Ana Porras, a Cornell Presidential Postdoctoral Fellow.
The project, done in collaboration with researchers from the Center for Studies of Sensory Impairment, Aging and Metabolism in Guatemala, grew out of a 2018 Cornell workshop on microbes and global health organized by Ilana Brito, assistant professor and the Mong Family Sesquicentennial Faculty Fellow in Biomedical Engineering in the College of Engineering, who led the team.
Brito already had a head start, having cultivated a cohort of microbiome samples from Fiji as part of her postdoctoral research.
"Across the world, you have a tremendous amount of diverse organisms in the gut microbiome. Those differences are way bigger than what we see in, for example, the U.S. population," Brito said. "There is a great need for understanding the specific causal roles those organisms play in health or promoting disease."
A range of factors can influence disparities in gut microbiome composition, from genetics and diet to antibiotic use, sanitation infrastructure and exposure to infectious diseases. It can be difficult to control for so many variables, so the researchers took microbiome samples from the three global populations and put them in 30 germ-free mice, then exposed them to Citrobacter rodentium, a model for severe intestinal infections like E. coli, to see how they responded.
"You get very different immune responses of these microbiomes in these different mice that led them to be either more or less resilient to infection," said Brito, who noted that Guatemala microbiota proved most resistant, followed by the U.S., then Fiji. "The interesting thing was they are exhibiting these differences in resilience to infection in a very short time. This isn't something that's happening over generations."
The researchers also exposed the mice to Listeria monocytogenes, but did not see similar responses, which suggests the microbiome is affecting certain attributes of the immune system and not others, Brito said.
In a follow-up experiment, the researchers co-housed mice with microbiomes from the three global regions and exposed them to C. rodentium. Because mice consume each other's stool, microbiota pass between them, essentially mimicking the way a human's microbiota composition can change when they travel, change their diet or permanently immigrate. The researchers found that less resilient mice benefited from the sharing of microbiota.
"It made the susceptible mice more resilient," Brito said. "So I think what these kind of experiments showed us was you can alter this immune background that comes from these bacteria in the gut, even on short time spans. There might be other things that can't be manipulated, like different populations that have genetic differences that make them more or less susceptible, but this microbiome phenotype is something that is more malleable."
The ability to transfer resistance to infection demonstrates the potential of harnessing the microbiome for therapeutic treatments.
"I think the broader point is that we should be studying the health effects of microbes that we find outside of our backyard," Brito said. "How could we improve people's health with microbiome interventions throughout the world, for different types of health problems? It warrants a global view."
Co-authors include research support specialist Qiaojuan Shi and doctoral student Hao Zhou; Rowan Callahan '18; and researchers from the Center for Studies of Sensory Impairment, Aging and Metabolism (CeSSIAM) in Guatemala.
More information: Ana Maria Porras et al, Geographic differences in gut microbiota composition impact susceptibility to enteric infection, Cell Reports (2021). DOI: 10.1016/j.celrep.2021.109457
Journal information: Cell Reports
Provided by Cornell University