Scientists can detect brain tumours using a simple urine or blood plasma test
Researchers from the Cancer Research UK Cambridge Institute have developed two tests that can detect the presence of glioma, a type of brain tumor, in patient urine or blood plasma.
The team say that a test for detecting glioma using urine is the first of its kind in the world.
Although the research, published in EMBO Molecular Medicine, is in its early stages and only a small number of patients were analyzed, the team say their results are promising.
The researchers suggest that in the future, these tests could be used by GPs to monitor patients at high risk of brain tumors, which may be more convenient than having an MRI every three months, which is the standard method.
When people have a brain tumor removed, the likelihood of it returning can be high, so they are monitored with an MRI scan every three months, which is followed by biopsy.
Blood tests for detecting different cancer types are a major focus of research for teams across the world, and there are some in use in the clinic. These tests are mainly based on finding mutated DNA, shed by tumor cells when they die, known as cell-free DNA (cfDNA).
However, detecting brain tumor cfDNA in the blood has historically been difficult because of the blood-brain-barrier, which separates blood from the cerebrospinal fluid (CSF) that surrounds the brain and spinal cord, preventing the passage of cells and other particles, such as cfDNA.
Researchers have previously looked at detecting cfDNA in CSF, but the spinal taps needed to obtain it can be dangerous for people with brain tumors so are not appropriate for patient monitoring.
Scientists have known that cfDNA with similar mutations to the original tumor can be found in blood and other bodily fluids such as urine in very low levels, but the challenge has been developing a test sensitive enough to detect these specific mutations.
The researchers, led by Dr. Florent Mouliere who is based at the Rosenfeld Lab of the Cancer Research UK Cambridge Institute and at the Amsterdam UMC, and Dr. Richard Mair, who is based at Cancer Research UK Cambridge Institute and the University of Cambridge developed two approaches in parallel to overcome the challenge of detecting brain tumor cfDNA.
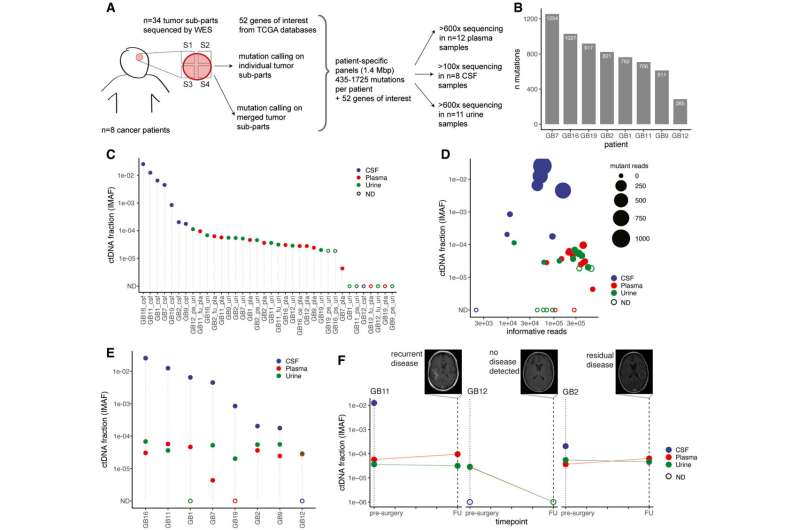
The first approach works for patients who have previously had glioma removed and biopsied. The team designed a tumor-guided sequencing test that was able to look for the mutations found in the tumor tissue within the cfDNA in the patient's urine, CSF, and blood plasma.
A total of eight patients who had suspected brain tumors based on MRIs were included in this part of the study. Samples were taken at their initial brain tumor biopsies, alongside CSF, blood and urine samples.
By knowing where in the DNA strand to look, the researchers found that it was possible to find mutations even in the tiny amounts of cfDNA found in the blood plasma and urine.
The test was able to detect cfDNA in 7 out of 8 CSF samples, 10 out of the 12 plasma blood samples and 10 out of the 16 urine samples.
For the second approach the researchers looked for other patterns in the cfDNA that could also indicate the presence of a tumor, without having to identify the mutations.
They analyzed 35 samples from glioma patients, 27 people with non-malignant brain disorders, and 26 healthy people. They used whole genome sequencing, where all the cfDNA of the tumor is analyzed, not just the mutations.
They found in the blood plasma and urine samples that fragments of cfDNA, which came from patients with brain tumors were different sizes than those from patients with no tumors in CSF. They then fed this data into a machine learning algorithm which was able to successfully differentiate between the urine samples of people with and without glioma.
The researchers say that while the machine learning test is cheaper and easier, and a tissue biopsy from the tumor is not needed, it is not as sensitive and is less specific than the first tumor-guided sequencing approach.
MRIs are not invasive or expensive, but they do require a trip to the hospital, and the three-month gap between checks can be a regular source of anxiety for patients.
The researchers suggest that their tests could be used between MRI scans, and could ultimately be able to detect a returning brain tumor earlier.
The next stage of this research will see the team comparing both tests against MRI scans in a trial with patients with brain tumors who are in remission to see if it can detect if their tumors are coming back at the same time or earlier than the MRI. If the tests prove that they can detect brain tumors earlier than an MRI, then the researchers will look at how they can adapt the tests so they could be offered in the clinic, which could be within the next ten years.
"We believe the tests we've developed could in the future be able to detect a returning glioma earlier and improve patient outcomes," said Mair. "Talking to my patients, I know the three-month scan becomes a focal point for worry. If we could offer a regular blood or urine test, not only will you be picking up recurrence earlier, you can also be doing something positive for the patient's mental health."
Michelle Mitchell, Chief Executive of Cancer Research UK said, "While this is early research, it's opened up the possibility that within the next decade we could be able to detect the presence of a brain tumor with a simple urine or blood test. Liquid biopsies are a huge area of research interest right now because of the opportunities they create for improved patient care and early diagnosis. It's great to see Cancer Research UK researchers making strides in this important field."
Sue Humphreys, from Wallsall, a brain tumor patient, said: "If these tests are found to be as accurate as the standard MRI for monitoring brain tumors, it could be life changing.
If patients can be given a regular and simple test by their GP, it may help not only detect a returning brain tumor in its earliest stages, it can also provide the quick reassurance that nothing is going on which is the main problem we all suffer from, the dreaded Scanxiety.
The problem with three-monthly scans is that these procedures can get disrupted by other things going on, such as what we have seen with the COVID pandemic. As a patient, this causes worry as there is a risk that things may be missed, or delayed, and early intervention is the key to any successful treatment."
More information: Florent Mouliere et al, Fragmentation patterns and personalized sequencing of cell‐free DNA in urine and plasma of glioma patients, EMBO Molecular Medicine (2021). DOI: 10.15252/emmm.202012881