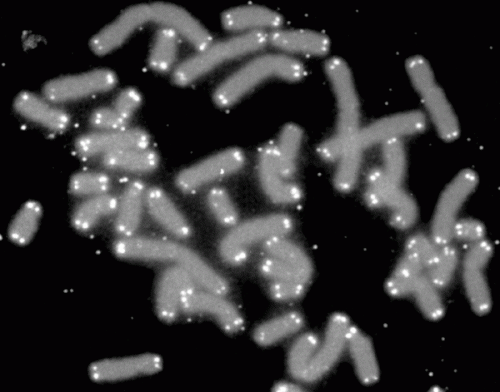
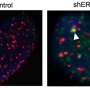
Human chromosomes (grey) capped by telomeres (white). Credit: PD-NASA; PD-USGOV-NASA
Research published in Science Signaling identifies new ways that telomeres regulate the aggressiveness and survival of tumors, making them potentially vulnerable therapeutic targets for killing cancer. The discovery was made by a team of researchers at Case Comprehensive Cancer Center and Case Western Reserve School of Medicine while studying how cancer cells switch telomere regulation and maintenance mechanisms during disease development and response to therapy.
Telomeres are the ends of chromosomes made of non-coding DNA. When normal cells divide, their telomeres become shorter until the cell can no longer divide. However, cancer cells can keep their telomeres long, extending their life indefinitely by activating one of two processes, either telomerase or the Alternative Lengthening of Telomeres (ALT) pathway.
The team, led by William Schiemann, the Goodman-Blum Professor in Cancer Research, delineated a pathway by which ALT is regulated—a mechanism that has not been well understood before. In addition, the team discovered the machinery that regulates ALT not only oversees telomere length but also functions in the cytoplasm to regulate signaling pathways associated with disease progression, cancer stem cells and chemoresistance. Their findings lead them to believe it is possible to target these new pathways to alleviate different cancers.
"This may be a unique opportunity to treat a tumor one way based on its predominant maintenance mechanism, knowing that when they develop resistance and flip to the other maintenance mechanism that we'll be able to hit them with the highly effective treatment," said Schiemann.
Though the research is still in the lab investigation stage, in the future, Schiemann said he would like to move to a proof-of-concept phase one clinical trial to test the patient's sensitivity to these inhibitors. Ideally, Schiemann said that therapeutic targeting of telomere maintenance mechanisms, particularly in adjuvant settings, has the potential to eliminate disease recurrence and metastatic relapse.
More information: Nathaniel J. Robinson et al, SLX4IP promotes RAP1 SUMOylation by PIAS1 to coordinate telomere maintenance through NF-κB and Notch signaling, Science Signaling (2021). DOI: 10.1126/scisignal.abe9613
Journal information: Science Signaling
Provided by Case Western Reserve University