Credit: Ecole Polytechnique Federale de Lausanne
Mesomelic dysplasias are a group of rare genetic conditions characterized by extreme shortening of the long bones in the arms and legs. Despite several decades of research on these conditions, the exact cause of mesomelic dysplasia has remained unclear. "It was a cold case," says Denis Duboule, a professor of Developmental Genetics and Genomics at EPFL. Now, researchers in Duboule's lab may have finally cracked the mystery of how mesomelic dysplasia develops.
Genetic analyses on people with mesomelic dysplasia have revealed that many individuals displaying short and bent forearm bones have a chromosomal rearrangement involving a region that contains the HOXD gene cluster—a collection of genes that are activated during embryonic development in different parts of the body, telling cells and tissues what to become. But none of the individuals analyzed had mutations affecting the HOXD genes themselves, Duboule says, suggesting that the limb deformities are the result of mutations interfering with the regulation of HOXD gene expression during early limb development.
However, much remained unknown about the mechanisms underlying the faulty regulation of HOXD genes. Studying such things in people is difficult, so Christopher Chase Bolt, a postdoc in Duboule's lab, turned to mice.
In the DNA, HOX genes line up in the same order as they are expressed in a developing embryo: first, the genes that regulate the formation of the shoulder; then, those that orchestrate the development of the arm; finally, those that guide the emergence of the hand and digits. Working in mice, Bolt created a chromosomal rearrangement that inverts the orientation of the HOXD cluster.
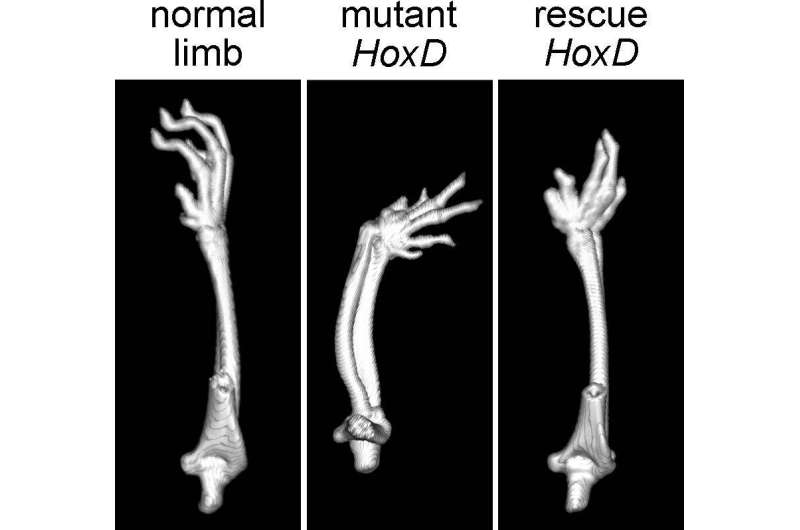
As a result of this inversion, the last gene of the cluster, called HOX13—which regulates the formation of the hand and digits, is located where the gene that orchestrates the development of the forearm typically is. Mice with this chromosomal rearrangement express HOX13 earlier during development than do control mice, and they display a mild form of mesomelic dysplasia, the researchers found. That's because HOX13 signals that the limb has reached its end and must stop growing, Bolt says, so having the gene expressed too early leads to extremely short extremities.
Next, Bolt and his colleagues used CRISPR-Cas9 gene-editing technique to engineer mice that had an inverted HOXD cluster but lacked the HOX13 gene. These animals did not have any limb deformities, suggesting that the faulty regulation of HOX13 expression is the main culprit for mesomelic dysplasia. "When you invert the cluster, HOX13 is expressed in the forearm and it results in mesomelic dysplasia," Duboule says. "But when you kill the gene, you get no problem—you actually rescue the phenotype." The study, published in Nature Communications, is the first to directly show the role of HOX13 misregulation in mesomelic dysplasia.
Because HOX genes are remarkably similar across disparate animals—from fruit flies to mice to humans, the findings have relevance for many people with mesomelic dysplasia. Indeed, the mechanism identified offers a molecular explanation for all cases of human mesomelic dysplasias involving HOX genes reported so far, Duboule says. "Now, each time a human geneticist reports this type of mesomelic dysplasias in humans, we can explain it through this conceptual framework," he says. "For us, the case is closed."
But Duboule's work continues: the team aims to figure out how HOX genes are controlled and how their activities determine where a limb starts and where it ends—in other words, how these 'architect' genes shape the body.
More information: Christopher Chase Bolt et al, Mesomelic dysplasias associated with the HOXD locus are caused by regulatory reallocations, Nature Communications (2021). DOI: 10.1038/s41467-021-25330-y
Journal information: Nature Communications
Provided by Ecole Polytechnique Federale de Lausanne