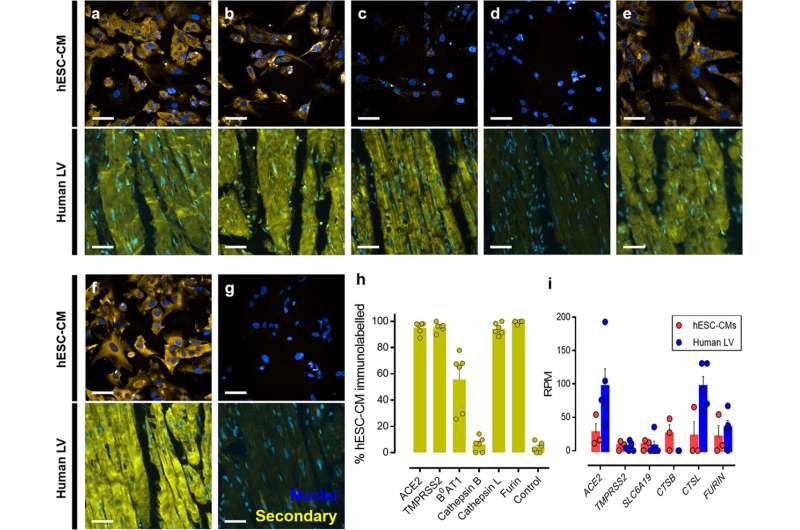
Fig. 1: Detection of host cell proteins and genes associated with SARS-CoV-2 viral infection. a–f Representative fluorescent confocal images (n = 3 independent experiments performed in duplicate) of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) (upper) and representative fluorescent images (n = 6 from 6 different donors) of human left ventricle (human LV) tissue sections (lower). Both cells and tissue were fixed with 4% formaldehyde and immunolabelled with primary antibodies raised against ACE2 a, TMPRSS2 b, B0AT1 c, cathepsin B d, cathepsin L e, and furin f, before visualization with secondary antibody conjugated to Alexa Fluor 555 (yellow) and Hoechst 33342 nuclear marker (blue). g shows control cells (upper) and tissue (lower) treated with secondary antibody only and Hoechst 33342 nuclear marker. Scale bars show 50 μm. h Graphical data showing the percentage of the observed hESC-CM population positively immunolabelled (above background) after visualization with a secondary antibody targeting primary antibodies raised against the outlined protein targets. i Graphical data showing the reads per million (RPM) ± SEM for expression of viral entry and processing genes in hESC-CMs (n = 3 replicates across three distinct differentiations) and human left ventricle (n = 5 individuals). SLC6A19, CTSB, and CTSL are the genes that encode B0AT1, cathepsin B, and cathepsin L, respectively. All graphical data are mean±SEM, with individual data points indicated. Credit: DOI: 10.1038/s42003-021-02453-y
Cambridge scientists have grown beating heart cells in the lab and shown how they are vulnerable to SARS-CoV-2 infection. In a study published in Communications Biology, they used this system to show that an experimental peptide drug called DX600 can prevent the virus entering the heart cells.
The heart is one the major organs damaged by infection with SARS-CoV-2, particularly the heart cells, or "cardiomyocytes," which contract and circulate blood. It is also thought that damage to heart cells may contribute to the symptoms of long COVID.
Patients with underlying heart problems are more than four times as likely to die from COVID-19, the disease caused by SARS-CoV-2 infection. The case fatality rate in patients with COVID-19 rises from 2.3% to 10.5% in these individuals.
To gain entry into our cells, SARS-CoV-2 hijacks a protein on the surface of the cells, a receptor known as ACE2. Spike proteins on the surface of SARS-CoV-2—which give it its characteristic "corona"-like appearance—bind to ACE2. Both the spike protein and ACE2 are then cleaved, allowing genetic material from the virus to enter the host cell. The virus manipulates the host cell's machinery to allow itself to replicate and spread.
A team of scientists at the University of Cambridge has used human embryonic stem cells to grow clusters of heart cells in the lab and shown that these cells mimic the behavior of the cells in the body, beating as if to pump blood. Crucially, these model heart cells also contained the key components necessary for SARS-CoV-2 infection—in particular, the ACE2 receptor.
Working in special biosafety laboratories and using a safer, modified synthetic ("pseudotyped') virus decorated with the SARS-CoV-2 spike protein, the team mimicked how the virus infects the heart cells. They then used this model to screen for potential drugs to block infection.
Credit: University of Cambridge
Dr. Sanjay Sinha from the Wellcome-MRC Cambridge Stem Cell Institute said: "Using stem cells, we've managed to create a model which, in many ways, behaves just like a heart does, beating in rhythm. This has allowed us to look at how the coronavirus infects cells and, importantly, helps us screen possible drugs that might prevent damage to the heart."
The team showed that some drugs that targeted the proteins involved in SARS-CoV-2 viral entry significantly reduced levels of infection. These included an ACE2 antibody that has been shown previously to neutralize pseudotyped SARS-CoV-2 virus, and DX600, an experimental drug.
DX600 is an ACE2 peptide antagonist—that is, a molecule that specifically targets ACE2 and inhibits the activity of peptides that play a role in allowing the virus to break into the cell.
DX600 was around seven times more effective at preventing infection compared to the antibody, though the researchers say this may be because it was used in higher concentrations. The drug did not affect the number of heart cells, implying that it would be unlikely to be toxic.
Professor Anthony Davenport from the Department of Medicine and a fellow at St Catharine's College, Cambridge said: "The spike protein is like a key that fits into the 'lock' on the surface of the cells—the ACE2 receptor—allowing it entry. DX600 acts like gum, jamming the lock's mechanism, making it much more difficult for the key to turn and unlock the cell door.
"We need to do further research on this drug, but it could provide us with a new treatment to help reduce harm to the heart in patients recently infected with the virus, particularly those who already have underlying heart conditions or who have not been vaccinated. We believe it may also help reduce the symptoms of long COVID."
More information: Thomas L. Williams et al, Human embryonic stem cell-derived cardiomyocyte platform screens inhibitors of SARS-CoV-2 infection, Communications Biology (2021). DOI: 10.1038/s42003-021-02453-y
Journal information: Communications Biology
Provided by University of Cambridge