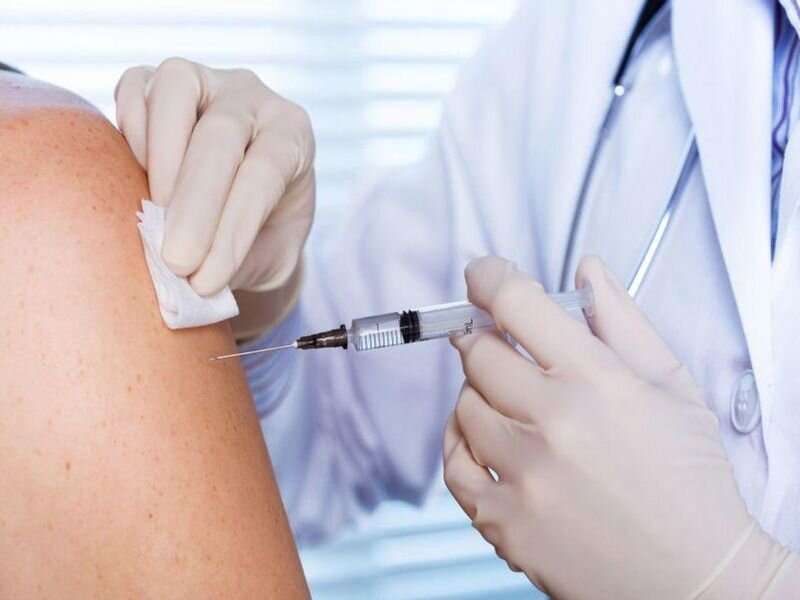
(HealthDay)—Most highly allergic individuals can be safely immunized against COVID-19, according to a study published online Aug. 31 in JAMA Network Open.
Ronen Shavit, M.D., from Sheba Medical Center in Tel Hashomer, Israel, and colleagues describe immunization of highly allergic individuals with the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine in a prospective cohort study conducted from Dec. 27, 2020, to Feb. 22, 2021. A total of 8,102 patients with allergies underwent risk assessment using an algorithm that included a detailed questionnaire; 429 patients were considered highly allergic and immunized under medical supervision.
The researchers found that 97.9 percent of the patients from the highly allergic group had no immediate allergic event after the first dose of the BNT162b2 vaccine, while 1.4 and 0.7 percent developed minor allergic responses and had anaphylactic reactions, respectively. During the study period, 50.8 percent of the highly allergic patients received the second BNT162b2 vaccine dose, 98.2 and 1.8 percent of whom had no allergic reactions and minor allergic reactions, respectively. Other immediate and late reactions were comparable to those seen in the general population, with the exception of delayed itch and skin eruption, which occurred more often in allergic patients.
"We enabled immunization of most patients with allergies by using a simple algorithm that included a referral center, a risk assessment questionnaire, and a safe environment for immunization of highly allergic patients with observation after immunization," the authors write. "This algorithm can be implemented in any medical setting to allow immunization for all."
One author disclosed financial ties to Teva and Pfizer.
More information:
Abstract/Full Text
Editorial
Journal information: JAMA Network Open
Copyright © 2021 HealthDay. All rights reserved.