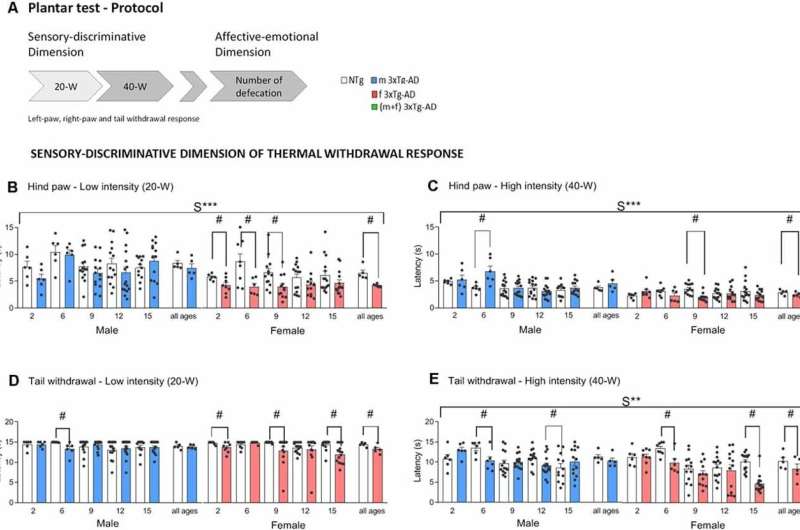
Figure 1. Schematic representation of the plantar test of thermal stimuli protocol used. Sensory-discriminative dimension of thermal withdrawal response in male and female 3xTg-AD and age-matched NTg mice with normal aging. (A) At low-20W and high-40W active intensity, infrared light stimuli were applied underneath the plantar surface of the mice’s hind paws (both right and left) and tail every 10 min. Paw (PWL) and tail (TWL) withdrawal latency were recorded for a sensory-discriminative dimension and the total number of defecations for an affective-emotional dimension. (B) Hind paw withdrawal responses at low-intensity 20-W (C) and high-intensity 40-W (D). Tail withdrawal at low-intensity 20-W (E) and high-intensity 40-W. Groups: NTg, non-transgenic mice (open bar); 3xTg-AD, transgenic mice (male “black,” female “red” bar). Error bars represent mean ± SEM. Statistics: Sex effect, S***, p < 0.001 or S** p < 0.01. Student t-test comparisons are shown in the graphs as #p < 0.05 vs. the NTg-group. Credit: DOI: 10.3389/fnagi.2021.683412
The increase in number of people at very advanced ages, in which several chronic diseases associated with pain can converge, make it of interest to research the regulatory mechanisms for the central nervous system which can react against a painful external stimulus. Problems associated with burn injuries may be of relevance in the daily life of older adults, but in people with dementia, exposure to high temperatures poses a significantly increased risk of burns.
Researchers at the UAB have explored the body's capacity to detect pain caused by a harmful external stimulus and the sensitivity to this pain in mice with different levels of cognitive deterioration caused by Alzheimer's disease, by exposing them to heat sources. The research studied the reaction of the animals from shortly after birth until old age, in phases imitating asymptomatic to advanced neuropathological stages of the disease. Conducted by Professor Lydia Giménez-Llort and Doctor Toni Cañete, both researchers at the Department of Psychiatry and Forensic Medicine and at the Institut de Neurociències (INc), the results of the study were recently published in the journal Frontiers in Aging Neuroscience.
The study demonstrates that sensitivity and response to pain is maintained in all stages of the disease. And for the first time, it characterizes differences according to age and gender with regards to sensory and emotional responses: females are more sensitive to pain, while males are more emotionally affected by it (distress, anxiety, and fear). Researchers also detected false-negative results in the "blind to sex and age" analysis, and therefore alert of the need to conduct independent studies with female and male mice.
"Our results, alongside other previous results we obtained when observing reactions to cold stimuli, verify that sensitivity in the nociceptive system exists even in the most advanced stages of Alzheimer's disease, and this reveals phenotypic variance in the disease in which the fundamental mechanisns involved should be studied according to age and sex," Lydia Giménez-Llort points out. "Moreover, it backs up similar results obtained in previous studies conducted with dementia patients, thus validating the mice model used to study the behaviors and mechanisms related to the reaction of pain in these individuals and possible new treatments," she adds.
"The study also will be useful to advance in preclinical trials on managing pain in dementia patients under the perspective of gender-based medicine, with the objective of developing pain treatments that are gender-specific," says Toni Cañete.
To conduct the study, researchers tested mice with thermal sensitivity tests similar to those conducted with humans suffering from cognitive deterioration, with the aim of observing psychobiological responses.
More information: Toni Cañete et al, Preserved Thermal Pain in 3xTg-AD Mice With Increased Sensory-Discriminative Pain Sensitivity in Females but Affective-Emotional Dimension in Males as Early Sex-Specific AD-Phenotype Biomarkers, Frontiers in Aging Neuroscience (2021). DOI: 10.3389/fnagi.2021.683412
Provided by Autonomous University of Barcelona