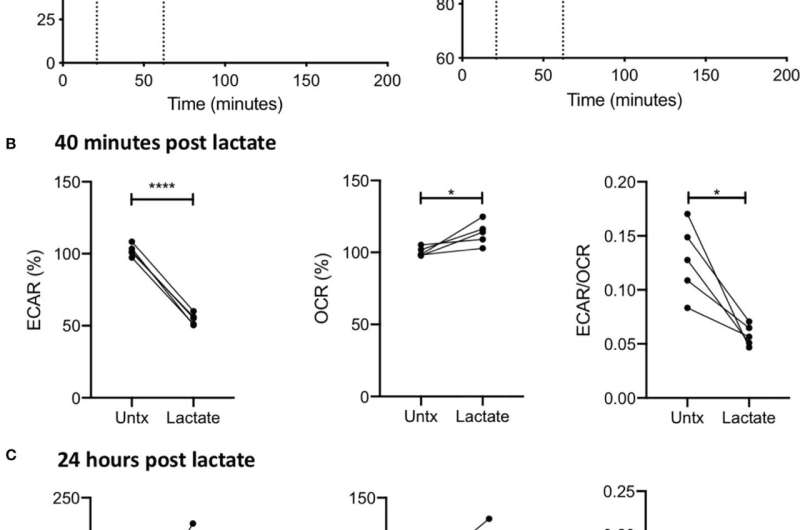
Figure 1. Lactate induces metabolic changes in human MDM. PBMC were isolated from buffy coats and MDM were adherence purified for 7 days in 10% human serum. MDM were gently scraped and seeded on Seahorse culture plates prior to analysis in the Seahorse XFe24 Analyzer. Lactate (25 mM) was either added to the cells through the Seahorse Analyzer ports (A, B) or 24 hours prior to analysis (C). Correction for differences in cell density was achieved by % comparison to the basal ECAR and OCR for the real-time analysis and by % comparison to the untreated MDM after crystal violet normalization for the analysis 24 hours post treatment. The ECAR and OCR were recorded approximately every 9 minutes. After 30 minutes, the SeahorseXFe24 Analyzer injected lactate or control medium (untreated; untx) into assigned wells. The ECAR and OCR readings were then continually sampled in real time. The time-course graphs illustrate the ECAR and OCR (A) of human MDM in response to treatment with lactate. At the time point indicated, 40 mins after administration, the effect of lactate on ECAR, OCR and the ECAR/OCR ratio (B) were calculated (n=5 ± SD). 24 hours after treatment with lactate, the basal ECAR, OCR and the ECAR/OCR ratio (C) were calculated (n=4 ± SD). Statistical significance was determined using a paired t test; *P < 0.05, ****P < 0.0001. Credit: DOI: 10.3389/fimmu.2021.663695
Tuberculosis (TB) is still one of the biggest infectious killers in the world. Multiple drug resistant (MDR) TB has become a global health emergency, an emerging European crisis, and an important Irish public health concern. Many challenges remain in the global fight against this disease including a clinical need for effective treatments against MDR-TB.
Now a research team based in the Trinity Translational Medical Institute (TTMI) at St James's Hospital are offering fresh hope for improving treatments for what remains a deadly disease, by focusing on identifying potential host-directed therapies that can target a patient's own immune response to help them recover from TB.
The research team has previously discovered that human immune cells change the way they use energy when they become infected. This change results in the generation of lactate, which was once considered a waste product of metabolism. New work by the team has shown that lactate can influence cells in the lung environment, to support immune defenses against infection and at the same time limit collateral damage to the delicate lung caused by excessive inflammation. Their study is published in leading journal, Frontiers in Immunology.
The research team is based in Professor Joseph Keane's TB Immunology lab at TTMI.
Dr. Cilian Ó Maoldomhnaigh undertook this research as part of a Ph.D. project funded by the National Children's Research Centre and the Royal City of Dublin Hospital Trust. He said, "Lactate has an immediate and striking effect on the metabolic function of human immune cells and reduces their ability to change their metabolism in response to subsequent infection. This has the knock-on effect of reducing inflammation, which can cause damage in the lung. We've also found that lactate can promote a cell's waste removal processes, allowing it to effectively dispose of the infection."
This waste removal process, called "autophagy," also plays a role in a wide range of other disease states such as Crohn's disease, cancer and heart disease, this indicates that lactate may hold therapeutic potential in many disease settings.
Senior author on the paper, Dr. Sharee Basdeo, Clinical Medicine, TTMI, said: "Our data indicate that aerosolised lactate delivered to the lungs may hold potential as a host-directed therapy for people battling lethal pneumonias—such as TB and COVID-19, where there is destruction of pulmonary tissue due to an unchecked pro-inflammatory response."
More information: Cilian Ó Maoldomhnaigh et al, Lactate Alters Metabolism in Human Macrophages and Improves Their Ability to Kill Mycobacterium tuberculosis, Frontiers in Immunology (2021). DOI: 10.3389/fimmu.2021.663695
Journal information: Frontiers in Immunology
Provided by Trinity College Dublin