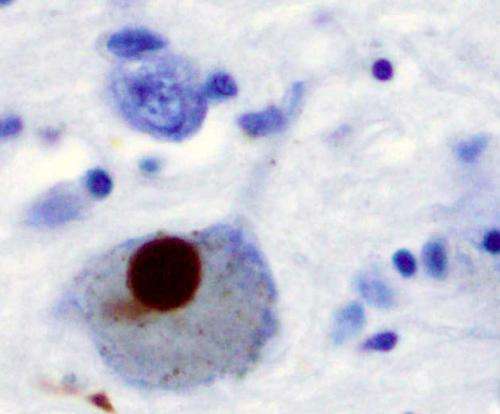
Immunohistochemistry for alpha-synuclein showing positive staining (brown) of an intraneural Lewy-body in the Substantia nigra in Parkinson's disease. Credit: Wikipedia
In late-stage Parkinson's disease, the drug levodopa becomes less effective in treating symptoms because of the inexorable loss of dopamine-releasing neurons. But a new Northwestern Medicine preclinical study shows a gene therapy targeting the small brain region where these neurons reside, the substantia nigra, substantially boosts the benefits of levodopa.
The gene therapy restored the ability of neurons in the substantia nigra to convert levodopa to dopamine. In essence, this allowed levodopa to recreate the environment found in the healthy brain and eliminated the aberrant brain activity responsible for difficulty in moving.
In the same study, scientists also provide an explanation for why dopamine-releasing neurons are lost in the disease. Using advanced genetic tools, the authors show that damage to the powerplants inside dopamine-releasing neurons (mitochondria) is sufficient to trigger a sequence of events that faithfully recapitulates what happens to brain circuits in Parkinson's disease.
The findings in mice, which will be published Nov. 3 in Nature, may help identify humans in the earliest stages of Parkinson's disease, develop therapies to slow disease progression and treat late-stage disease.
The key new findings:
- Damage to the power plants in dopamine-releasing neurons is enough to cause Parkinson's disease. When these power plants (mitochondria) begin to shut down, the ability of neurons to do their jobs in the brain is compromised. Without a sufficient source of energy, neurons eventually wither and die. This finding opens a new path to develop therapies to protect the function of mitochondria.
- Contrary to the past 30 years of thinking, the emergence of the motor symptoms of Parkinson's disease requires the loss of dopamine release in a small region of the brain called the substantia nigra. This discovery also opens the door to new therapies for late-stage Parkinson's disease patients.
- Scientists demonstrated that a gene therapy targeting the substantia nigra effectively boosts the symptomatic benefit of levodopa.
"The development of effective therapies to slow or stop Parkinson's disease progression requires scientists know what causes it," said lead study author D. James Surmeier, chair of neuroscience at Northwestern University Feinberg School of Medicine. "This is the first time there has been definitive evidence that injury to mitochondria in dopamine-releasing neurons is enough to cause a human-like parkinsonism in a mouse.
"Whether mitochondrial damage was a cause or consequence of the disease has long been debated. Now that this issue is resolved, we can focus our attention on developing therapies to preserve their function and slow the loss of these neurons."
In addition to providing a clear target for disease-modifying therapies, the study provides a model of Parkinson's disease before clinical symptoms appear. The slow, progressive loss of dopamine-releasing neurons in the model allowed researchers to see what may be happening in the brain well before movement becomes difficult.
"This new 'human-like' model may help us develop tests that would identify people who are on their way to being diagnosed with Parkinson's disease in five or 10 years," Surmeier said. "Doing so would allow us to get them started early on therapies that could alter disease progression."
Other Northwestern authors are Patricia Gonzalez-Rodriguez, Enrico Zampese, Kristen Stout, Jamie Guzman, Ben Yang, Tatiana Tkatch, Ema Ilijic and Paul Schumacker.
More information: Dalton Surmeier, Disruption of mitochondrial complex I induces progressive parkinsonism, Nature (2021). DOI: 10.1038/s41586-021-04059-0. www.nature.com/articles/s41586-021-04059-0
Journal information: Nature
Provided by Northwestern University