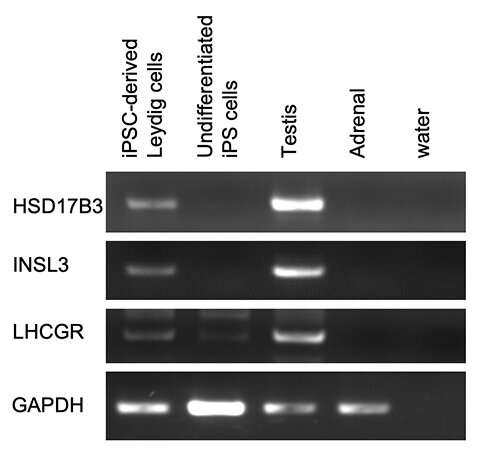
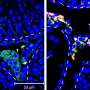
Figure 1: Expression of Leydig cell-specific genes. Credit: Kobe University
Researchers from Kobe University's Graduate School of Science, Technology and Innovation/Graduate School of Medicine have succeeded in generating Leydig cells from human iPS cells. Leydig cells are responsible for producing the male hormone (testosterone), and it is hoped that Leydig cell transplants could serve as a treatment for late-onset hypogonadism in the future.
The research group included Professor Aoi Takashi of the Department of iPS cell applications, and Researcher Ishida Takaki and Kobe University President Fujisawa Masato of the Division of Urology.
These research results were published in Endocrinology on September 21, 2021.
It is well known that middle-aged women suffer from perimenopausal symptoms around 10 years before the menopause due to declining levels of the female hormone estrogen. However, men also experience a decrease in hormone levels (testosterone) as they age, and recognized symptoms of this include depression, decline in sexual function (e.g. libido and erectile function), reduced muscle mass and reduced bone density, all of which can have a significant impact on patients' quality of life (QOL). It is said that anywhere between a few hundred thousand to over two million men in Japan have symptoms, however awareness remains low.
Currently, patients with late-onset hypogonadism (LOH) are treated with male hormone replacement therapies, however there are problems with these treatments. They require frequent hospital visits and do not restore the physiological functions that regulate hormone levels in the blood. In light of this, the researchers decided to try to generate testosterone-producing Leydig cells from human iPS cells (that have the ability to differentiate into all types of cells) in the hope that transplanting these induced cells into patients would provide a definitive treatment method for LOH.
-
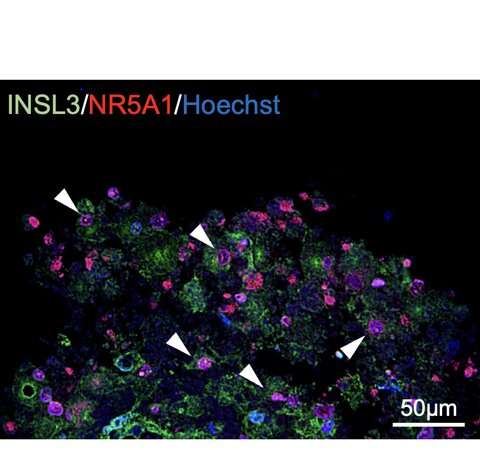
Figure 2: Expression of Leydig cell-specific genes.White arrowheads indicate double-positive cells. Credit: Kobe University
-
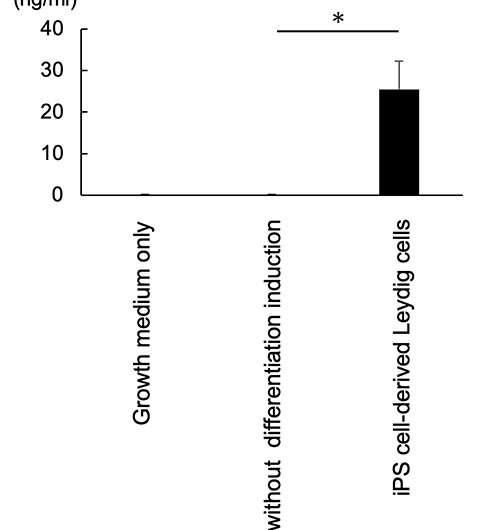
Figure 3: Production of testosterone by iPS cell-derived Leydig cells. Credit: Kobe University
-
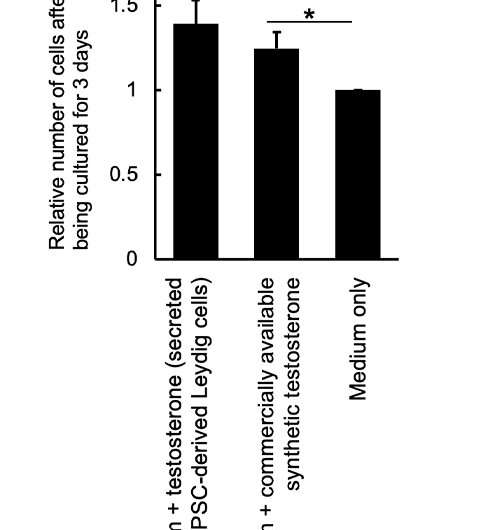
Figure 4: Testosterone produced by iPS cell-derived Leydig cells: enhanced proliferation of a testosterone-sensitive cell line (LNCap). Credit: Kobe University
Ishida et al. generated Leydig cells by expressing NR5A1 in male-derived human iPS cells. NR5A1 plays an important role in the development of the gonads and adrenal glands. The generated cells expressed genes specific to Leydig cells (Figures 1 and 2).
These iPS-generated Leydig cells produced the male hormone (testosterone) (Figure 3). A cell proliferation experiment was then conducted using LNCaP cells, which proliferate in response to testosterone. The researchers found that testosterone secreted from the iPS-derived Leydig cells enhanced LNCaP cell proliferation, indicating that the hormone was functional (Figure 4).
This research succeeded in generating Leydig cells from human iPS cells. It is thought that this achievement will lead to the future implementation of regenerative medicine in which Leydig cells produced from human iPS cells are transplanted into patients with LOH.
In addition, Leydig cells are known to be difficult to cultivate as they do not proliferate in vitro. Therefore, Leydig cells generated from human iPS cells could be useful in a wide variety of research, not just regenerative medicine.
More information: Takaki Ishida et al, Differentiation of Human Induced Pluripotent Stem Cells Into Testosterone-Producing Leydig-like Cells, Endocrinology (2021). DOI: 10.1210/endocr/bqab202
Journal information: Endocrinology
Provided by Kobe University