Clinical trial supports new self-administered rapid antigen test
When it comes to COVID-19, peace of mind is difficult to come by.
But thanks to new research compiled with data from UBC's first on-campus clinical study, a new self-administered rapid antigen test will soon be available in Canada. It's a tool that could help combat growing uncertainty, prevent transmission and potentially save lives.
The research was used by Quebec-based Roche Diagnostics in agreement with SD Biosensor Inc. to further validate a new SARS-CoV-2 Antigen Self Test Nasal, allowing users to get test results at home in about 15 minutes.
Principal investigator Dr. Sabrina Wong and fellow researchers—including investigators Dr. Marc Romney and Dr. Don Sin of UBC and Providence Health and UBC's Dr. Kristen Haase—surveyed 689 participants at UBC's Vancouver campus. It was a part of a larger study that looked at the effectiveness of self-administration of rapid antigen testing amongst asymptomatic adults in Canada.
In this Q&A, UBC school of nursing professor and associate director Dr. Wong shares more.
What is a rapid antigen test?
A rapid antigen test is a quick and easy tool to detect if someone has a high SARS-CoV-2 viral load, which means they are likely to transmit the virus to someone else. This type of screening test is intended to identify infected individuals not currently showing symptoms but who may be contagious, so that measures can be taken to prevent transmission.
How does this new test differ from others?
The Roche SARS-CoV-2 Antigen Self Test Nasal is one of the few rapid antigen tests approved for self-testing amongst both symptomatic and asymptomatic people.
It has a shelf life of 24 months and uses a nasal swab to collect the sample. This means the test can be widely available across supermarkets, drug stores and so on. This also means that the federal, provincial and territorial governments can now procure this rapid test and distribute to Canadians where there are higher risks and those who cannot buy them.
How did UBC's research contribute?
UBC conducted the clinical study, with two primary objectives: To test self-administration of a SARS-CoV-2 Antigen Self Test Nasal compared to application of this same test by a trained health professional, and to examine the sensitivity and specificity of the test in detecting infection amongst an asymptomatic population.
We found the results from the self-test versus the healthcare professional's gave near identical results. We also showed that this method has acceptable sensitivity (94.9 percent) when compared to the PCR test that detects the virus's genetic material.
What else did you find through UBC's pilot study?
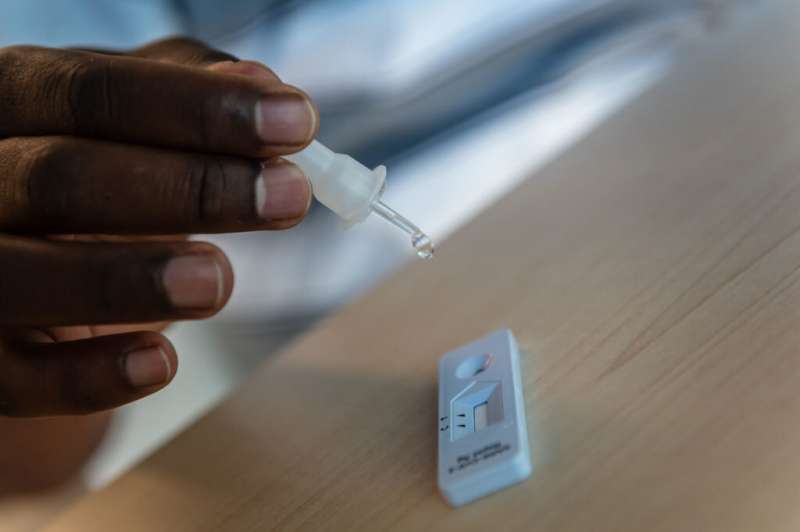
The biggest takeaway is that people tend to stick the nasal swab way too far up their nose. The nasal swab is meant to get mucosal cells just inside the nostril, so no need to stick it so far up.
Another takeaway is that rapid tests are an additional layer of protection for individuals, organizations and systems. People can be empowered to protect themselves and protect others by helping to stop future transmission. Most people would not knowingly spread an infectious disease.
Why is this approval important for Canadians?
In B.C. and most of Canada we've been painstakingly slow to embrace rapid antigen testing. As a society we have adopted many tools that previously would have been unthinkable: Masks, physical and social distancing and multiple vaccines. However, few British Columbians have access to these simple yet essential screening tests.
COVID-19 has disrupted our daily lives for nearly 21 months now. Initially the "finish line" was vaccination, but with the rise of this latest variant and pending fifth wave, that finish line keeps moving.
Rapid testing provides peace of mind to individuals and their contacts but it is just one layer of protection and needs to be used with other preventive measures, including physical distancing, masks and vaccination.