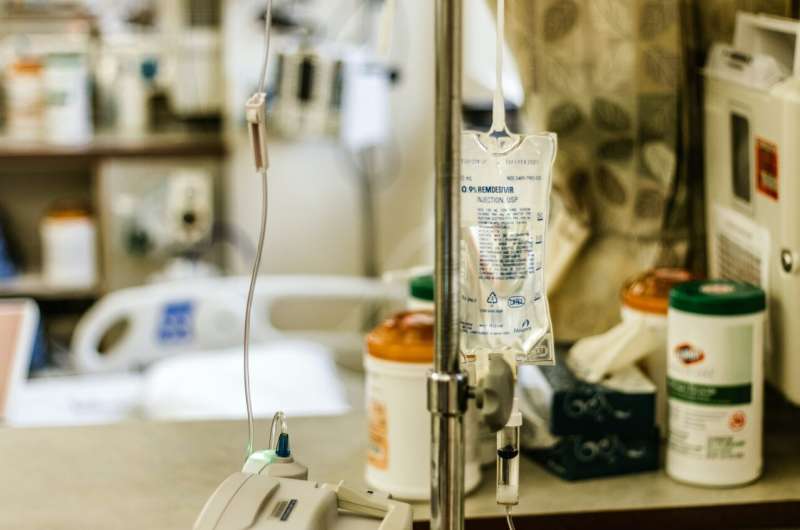
Credit: Unsplash/CC0 Public Domain
A three-day course of remdesivir results in a lower risk for hospitalization or death compered with placebo among nonhospitalized patients at high risk for COVID-19 progression, according to a study published online Dec. 22 in the New England Journal of Medicine.
Robert L. Gottlieb, M.D., Ph.D., from the Baylor University Medical Center in Dallas, and colleagues conducted a randomized trial involving nonhospitalized patients with COVID-19 with symptom onset within the previous seven days and with at least one risk factor for disease progression. Patients were randomly assigned to receive intravenous remdesivir (200 mg on day 1; 100 mg on days 2 and 3) or placebo (279 and 283 patients, respectively).
The researchers found that two patients in the remdesivir group and 15 in the placebo group had COVID-19-related hospitalization or death (0.7 versus 5.3 percent; hazard ratio, 0.13). By day 28, 1.6 and 8.3 percent of patients in the remdesivir and placebo groups, respectively, had a COVID-19-related medically attended visit (hazard ratio, 0.19); there were no deaths by day 28. Adverse events occurred in 42.3 and 46.3 percent of those in the remdesivir and placebo groups, respectively.
"In the campaign toward ending the COVID-19 pandemic, these data add yet another option to the armamentarium for the treatment of vulnerable patients who are at high risk for progression to severe COVID-19," the authors write.
The study was funded by Gilead Sciences, the manufacturer of remdesivir.
More information: Robert L. Gottlieb et al, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, New England Journal of Medicine (2021). DOI: 10.1056/NEJMoa2116846
Emily L. Heil et al, The Goldilocks Time for Remdesivir—Is Any Indication Just Right?, New England Journal of Medicine (2021). DOI: 10.1056/NEJMe2118579
Journal information: New England Journal of Medicine
Copyright © 2021 HealthDay. All rights reserved.