Credit: CC0 Public Domain
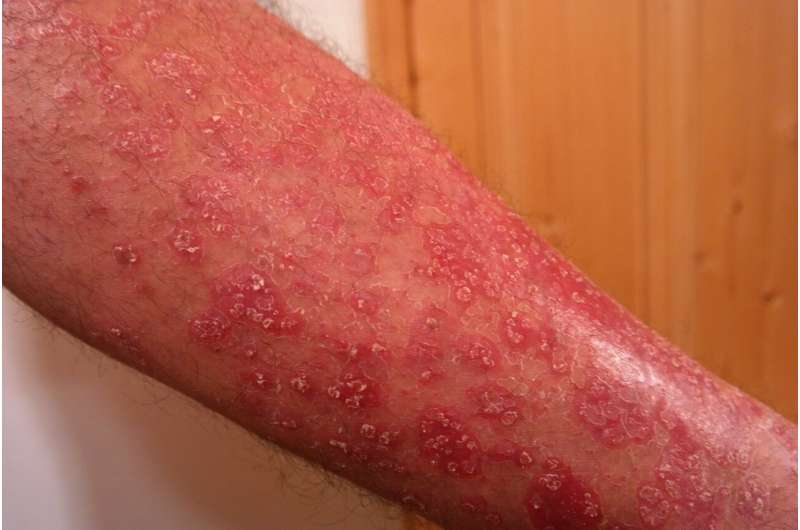
Generalized pustular psoriasis (GPP) is a rare, life-threatening skin condition for which there are no approved treatments. It is characterized by episodes of widespread eruptions of painful, sterile pustules (blisters of non-infectious pus). There is a high unmet need for treatments that can rapidly and completely resolve the signs and symptoms of GPP flares. Flares greatly affect a person's quality of life and can lead to hospitalization with serious complications, including heart failure, renal failure, sepsis, and death.
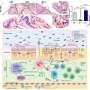
A clinical trial has shown that spesolimab is a novel, humanized, selective antibody that blocks the activation of the interleukin-36 receptor (IL-36R), a signaling pathway within the immune system shown to be involved in the pathogeneses of several autoimmune diseases, including generalized pustular psoriasis. The novel drug demonstrated rapid clearance of pustules in adult patients with generalized pustular psoriasis (GPP) experiencing a flare.
The study met the primary endpoint; 54% of patients had no visible pustules after a single dose of spesolimab, compared to 6% receiving a placebo at week one.
The bottom line appears to be that spesolimab is rapidly effective in the majority of patients within one week of its first intravenous infusion for patients suffering from generalized pustular psoriasis. This is significant because generalized pustular psoriasis is a life-threatening condition that compromises the integrity of the skin. Patients are frequently hospitalized and often die from sepsis or other complications.
The study's senior author, Mark Lebwohl, MD, said, "Generalized pustular psoriasis is a rare life-threatening condition in which the protective functions of the skin are lost, leaving patients vulnerable to loss of electrolytes, nutrients, and fluids for the skin, causing high output cardiac failure, sepsis, and even death. Until now, other treatments used for this condition have not been reliably effective and are often too slow for a condition that is so dangerous. Spesolimab will hopefully be the first approved treatment in the United States for generalized pustular psoriasis, and we will finally have a reliable, rapidly effective treatment for this devastating disease."
More information: Hervé Bachelez et al, Trial of Spesolimab for Generalized Pustular Psoriasis, New England Journal of Medicine (2021). DOI: 10.1056/NEJMoa2111563
Journal information: New England Journal of Medicine
Provided by The Mount Sinai Hospital