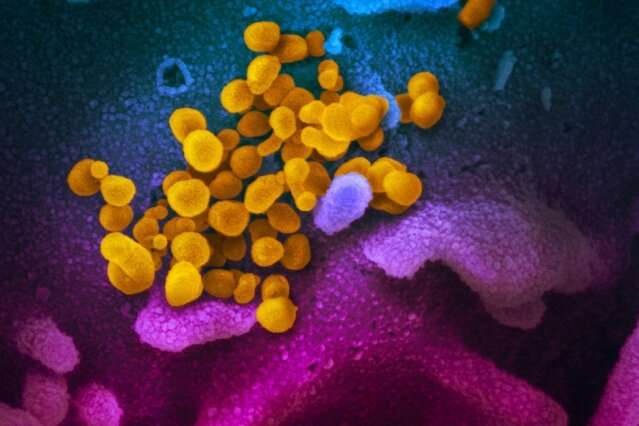
This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient, emerging from the surface of cells (blue/pink) cultured in the lab. Credit: NIAID-RML
EU regulators on Thursday recommended two new treatments against COVID-19 for use in the bloc.
The European Medicines Agency (EMA) said Kineret, an immunosuppressive produced by Swedish Orphan Biovitrum to treat inflammatory conditions, could "decrease lower airway damage, preventing development of severe respiratory failure".
It said also that GlaxoSmithKline's Xevudy drug had shown in a study that it "significantly reduces hospitalisation and deaths in patients with at least one underlying condition".
Kineret is already recommended by the EMA to treat various conditions, with the agency expanding its listing to cover COVID-19.
Its use is recommended for adults "with pneumonia requiring supplemental oxygen... who are at risk of developing severe respiratory failure", the EMA said in a statement.
Xevudy, developed by GSK with US firm Vir Biotechnology, is the third drug backed by the EMA that uses "monoclonal antibodies", which are proteins designed to attach to the spike protein of SARS-CoV-2, the virus that causes COVID.
The agency said Xevudy would be recommended for most patients aged 12 and above "who are at increased risk of the disease becoming severe".
© 2021 AFP