Human microRNA inhibits expression of pathogenic gene underlying facioscapulohumeral muscular dystrophy
Facioscapulohumeral muscular dystrophy (FSHD) is caused by aberrant expression of the DUX4 gene in skeletal muscles. Researchers at Nationwide Children's Hospital have recently demonstrated that an endogenous human microRNA, miR-675, inhibits DUX4 expression and protects muscles from DUX4-mediated cell death in a mouse model when administered via gene therapy. They also showed that the small molecule-based treatments that upregulate miR-675 inhibited DUX4 mRNA and DUX4-associated biomarkers in myotubes derived from patients with FSHD.
According to the researchers, the study, which was published in Nature Communications, is the first to demonstrate the use of small molecules to suppress a dominant disease gene using an RNA interference mechanism.
"The idea was to take advantage of endogenous molecules that every person already has in their muscles," says Nizar Saad, Ph.D., research scientist in the laboratory of Scott Harper, Ph.D., principal investigator at the Center for Gene Therapy at the Abigail Wexner Research Institute at Nationwide Children's. "Because we validated that this microRNA can directly target DUX4 mRNA, we developed two methods with which we can upregulate the microRNA levels."
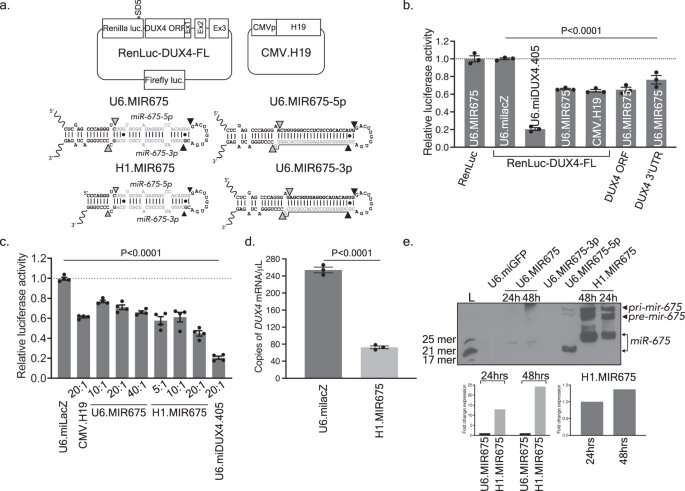
MicroRNAs are small noncoding RNAs (~22 nucleotides) that serve as post-transcriptional regulators of gene expression. Prior to the study, no endogenous microRNAs targeting DUX4 had been identified. Thus, Dr. Saad and colleagues used bioinformatics tools to predict which human microRNAs may target DUX4 mRNA and then empirically tested their abilities to silence DUX4expression. Among numerous candidates, the team found that miR-675 inhibited DUX4 expression in human kidney cells.
They then demonstrated that miR-675 could regulate DUX4expression in patient muscle cells, which naturally express DUX4and miR-675. The successful repression of DUX4 in human muscles cells justified further study of the therapeutic potential of miR-675 in a murine model of FSHD.
The researchers developed a viral vector-based gene therapy to deliver miR-675 to mice with FSHD and demonstrated that its delivery can inhibit DUX4-associated histopathology and protect muscles from DUX4-associated cell death.
Next, the team validated previous studies showing that cellular levels of miR-675 can be increased by treatment with either estrogen/progesterone or melatonin. They then used these small molecules to upregulate endogenous miR-675 and, in turn, decrease DUX4 and DUX4-responsive biomarkers in the human FSHD myotubes derived from patients.
"Gene therapy has the potential to permanently alter pathogenic gene expression in FSHD; however, in some cases, it may be desirable to use small molecules to modulate levels of miR-675 to regulate DUX4 expression and counteract its toxicity in muscle. Some of these molecules are already FDA-approved for other treatments and are readily accessible," explains Dr. Saad.
The study supports translation of miR-675 gene and drug therapies as prospective treatments for FSHD, and justifies identifying additional regulators that could be exploited for FSHD therapy.
"Our study also functions as a proof of principle—we aim to establish a strategy, where investigators can screen small molecule libraries to identify compounds that may modulate the expression of microRNAs of interest to develop additional RNAi-based small molecule therapies for FSHD and other dominant diseases," adds Dr. Saad.
More information: Nizar Y. Saad et al, Human miRNA miR-675 inhibits DUX4 expression and may be exploited as a potential treatment for Facioscapulohumeral muscular dystrophy, Nature Communications (2021). DOI: 10.1038/s41467-021-27430-1