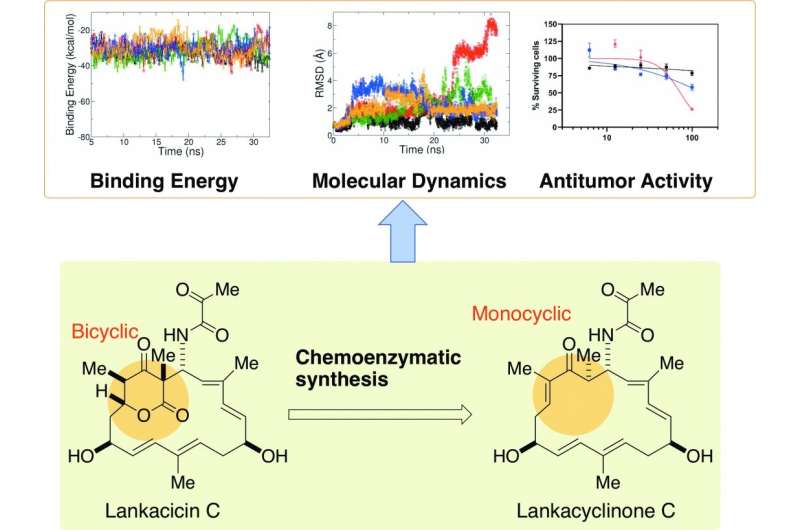
Chemoenzymatic synthesis and physico-chemical and biological functions of monocyclic polyketide compound, lankacyclinone C.Scientists from Hiroshima Univ., Univ. Toyama, Heliopolis Univ. (Egypt), and Univ. Alberta (Canada) revealed the functional importance of monocyclic polyketide, lankacyclinone C, for the antitumor activity. Credit: Kenji Arakawa, Hiroshima University
Garden soil houses a variety of bacteria and their natural byproducts—including one that may help halt tumor growth. Lankacidins are molecules that can be isolated from Strepomyces rochei, a common bacterium in soil. In addition to antimicrobial properties, a type of lankacidins, called lankacidin C, can inhibit tumor activity in various cancer cell lines, including leukemia, melanoma, ovarian and breast cancers. Lankacidin C offers a potential foundation on which to design anticancer drugs, but its structure is complicated and difficult to manipulate, according to an international research group. The same group recently identified where antitumor activity is housed on the molecule and has now used that information to simplify lankacidin as a potential starting point to engineer treatments.
They published their results on Jan. 1 in Bioorganic & Medicinal Chemistry.
"Lankacidins have potential antitumor agents, however, their structural modification has somewhat problem due to the presence of complex bicyclic ring in lankacidin antibiotics," said paper author Kenji Arakawa, associate professor at Hiroshima University's Graduate School of Integrated Sciences for Life. "Structural modification of lankacidin C, as a mother compound, would be an excellent starting point for enhancing antitumor activity through computational prediction."
The researchers preliminarily assessed how various components of lankacidin-group antibiotics may contribute to its antitumor activity using a computational model, finding that a structural ring of carbon atoms, called the delta-lactone ring, may not be essential. According to Arakawa, the implication was striking, since the ability to structurally modify lankacidins has been limited by the presence of the delta-lactone ring.
"In this study, we synthesized lankacyclinone C, a novel lankacidin C variant lacking the delta-lactone ring," Arakawa said. "In doing so, we solved one major issue of structural function in the lankacidin skeleton, the bicyclic structure of the delta-lactone ring, for antitumor activity."
They used a protein called Orf23 to convert the bicyclic structure with the delta-lactone ring to a monocyclic version. A computational model predicted that the resulting lankacyclinone C, with a simplified ring, would still prove cytotoxic to target cancer cells. Experimental results supported the prediction.
"Rather than bicyclic lankacidins, structurally simple and flexible monocyclic lankacidins may be better substrates for further structural redesigning to improve antitumor activity," Arakawa said.
According to Arakawa, the researchers plan to further investigate the rational design of molecular compounds with the goal of creating the ultimate antitumor agents.
More information: Rukman Muslimin et al, Chemoenzymatic synthesis, computational investigation, and antitumor activity of monocyclic lankacidin derivatives, Bioorganic & Medicinal Chemistry (2021). DOI: 10.1016/j.bmc.2021.116551
Provided by Hiroshima University