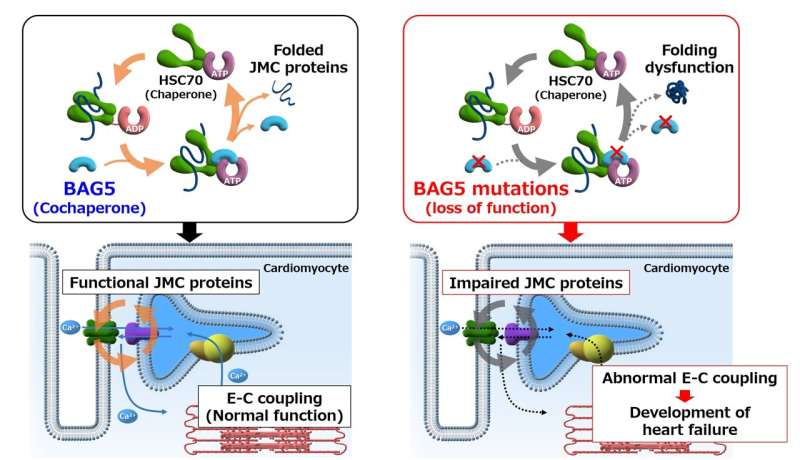
Loss of function mutations in the cochaperone protein BAG5 can lead to dilated cardiomyopathy and ultimately heart failure through an impaired quality control of junctional membrane complex (JMC) proteins. E-C coupling: Excitation-Contraction coupling. Credit: 2022 Hakui H et al., Science Translational Medicine
Researchers from Osaka University found a previously unknown gene mutation that can cause an incurable heart condition called dilated cardiomyopathy. This gene, BAC5, is important for the movement of calcium ions in the heart muscle and calcium ions are what drives the pumping of the heart. The good news is that the investigators also found a way to fix the mutation through a novel gene therapy approach, demonstrating a potential treatment for this devastating disease.
The heart is a tireless organ, beating an average of 100,000 times a day. However, conditions that stop the heart from pumping blood efficiently can cause serious problems and ultimately require a heart transplantation.
In a study published this month in the journal Science Translational Medicine, researchers from Osaka University have shown that a previously unknown mutation can lead to a condition called dilated cardiomyopathy, which is one of the main causes of heart failure.
Heart failure is an incurable condition where the heart is no longer able to meet the body's demands in terms of blood supply. It is one of the most common causes of death and it affects almost 40 million people worldwide, representing a huge public health problem. One of the main factors leading to heart failure is a disease called dilated cardiomyopathy (or DCM). DCM is characterized by dilation of the heart's chambers and a pumping disfunction. Previous research has shown that DCM is often inherited and has a genetic basis. However, for up to 80% of the familial DCM cases, we still don't know the genetic mutation causing the disease.
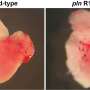
The research team identified a gene called BAG5 as a novel causative gene for DCM. First, they studied patients from different families, highlighting a correlation between loss of function mutations in the BAG5 gene and DCM. The researchers found that this mutation has a complete penetrance, meaning that 100% of the individuals presenting it will develop the disease. They then found in a mouse model of dilated cardiomyopathy that mice without BAG5 exhibited the same symptoms of human DCM, such as dilatation of the heart's chambers and irregular heart rhythm. This indicates that mutations that erase the function of BAG5 can cause cardiomyopathy.
"Here we showed that loss of BAG5 perturbs calcium handling in mouse cardiomyocytes," says Dr. Hideyuki Hakui, lead author of the study. BAG5 is important for calcium handling in the heart muscle cells, and calcium is essential for a regular rhythm and overall health of the cardiac muscle, explaining why a loss of BAG5 leads to cardiomyopathy.
"After demonstrating that BAG5 mutations led to loss of functional BAG5 protein," continues Dr. Yoshihiro Asano, senior author of the study, "we also showed that administration of an AAV9-BAG5 vector in a murine model could restore cardiac function. This finding suggests that gene therapy with adeno-associated viruses (AAV) should be further investigated as a possible treatment alternative to heart transplantation for patients who are BAG5 deficient." AAV gene therapy is an innovative form of therapy aimed at fixing mutated genes in diseases that have a genetic cause like DCM. Therefore, these findings pave the way for a potential precision medicine treatment based on gene therapy.
More information: "Loss-of-function mutations in the co-chaperone protein BAG5 cause dilated cardiomyopathy requiring heart transplantation," Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abf3274
Journal information: Science Translational Medicine
Provided by Osaka University