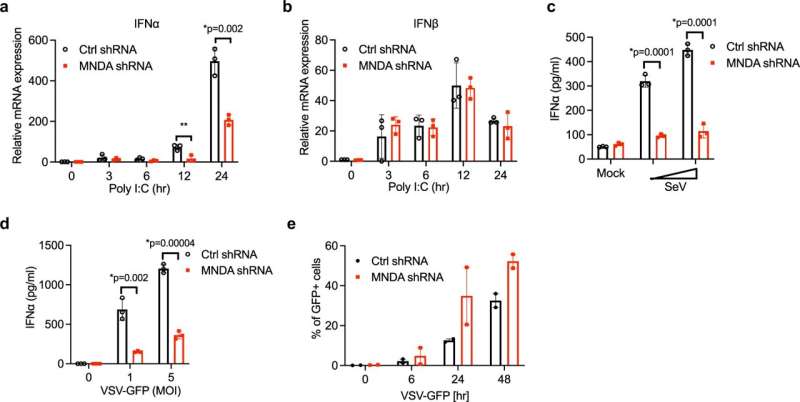
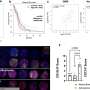
MNDA regulates dsRNA- and virus-stimulated IFNα induction in monocytes. a, b Quantitative PCR analysis of IFNα (a) and IFNβ (b) mRNA from THP-1 cells expressing control or MNDA shRNA transfected with poly(I:C) (2.5 μg/ml) for the indicated times. c, d Release of IFNα protein from THP-1 cells expressing control or MNDA shRNA and infected with Sendai virus (SeV) for 24 h (c) or with VSV-GFP at the indicated MOI for 48 h (d). e Analysis of GFP protein expression by flow cytometry, as a measure of viral replication, in THP-1 cells expressing control or MNDA shRNA infected with VSV-GFP at an MOI of 5 for the indicated times. Data are mean ± SD of triplicate samples and are representative of three independent experiments (a–d) or are mean of two experiments (e); two tailed unpaired Students t test; *p < 0.05 indicates significance compared to respective groups; ns indicates not significant. Credit: DOI: 10.1038/s41467-021-27701-x
Scientists have just slotted into place a big piece of the puzzle that explains how our blood cells mount their first line of defense against viruses. They hope this discovery will help them one day better control the response to either boost it or calm it down as appropriate.
The scientists behind the discovery include Andrew Bowie, Professor of Innate Immunology at Trinity College Dublin, who is based in the Trinity Biomedical Sciences Institute, and Drs Lili Gu and David Casserly (formerly at Trinity as postdoctoral and Ph.D. researchers respectively).
Their findings, which provide a target for new therapies that could improve anti-viral responses in some patients and reduce autoimmune problems in others when immune responses run out of control, have just been published in the journal Nature Communications.
Interferons and MNDA—a major piece of the puzzle
"Interferons" are key proteins that tell our immune systems when viruses, germs or cancer cells are in our bodies. Type I interferons are produced when the innate immune system senses the presence of a virus. In such circumstances interferons trigger a complex chain of events in which other cells are kicked into gear to "interfere with" and fight those invaders.
Scientists don't fully understand how certain links in that chain of events are controlled—making it difficult to stimulate or suppress an immune response with therapeutic interventions—but this new research has provided key new insights into the process.
The scientists have discovered that another protein, myeloid cell nuclear differentiation antigen (MNDA), is needed for Type I interferon production from human blood cells in response to viruses. Their work shows that MNDA regulates a transcription factor, IRF7, which in essence drives Type I interferon production.
Professor Andrew Bowie said, "We have been interested in better understanding how Type I interferons are produced from blood cells because they are required to fight viruses, and because too much of them—when the production process runs out of control, for example—contributes to nasty autoimmune diseases such as interferonopathies.
"There is a family of proteins called PYHIN proteins which we have been working on for some time, as they are implicated in regulating innate immunity. To our surprise, one such PYHIN protein, called MNDA, turned out to be a big missing piece in the puzzle in understanding how type I interferon production is sustained, which makes this discovery all the more exciting.
"If we can learn how to manipulate MNDA's activity it could be really beneficial—on one hand to boost an interferon response during a viral infection, for example upon COVID-19 infection, or on the other hand to supress interferon production and treat an autoimmune disorder."
The scientists are currently examining how MNDA contributes to innate immune responses to COVID-19.
More information: Lili Gu et al, Myeloid cell nuclear differentiation antigen controls the pathogen-stimulated type I interferon cascade in human monocytes by transcriptional regulation of IRF7, Nature Communications (2022). DOI: 10.1038/s41467-021-27701-x
Journal information: Nature Communications
Provided by Trinity College Dublin