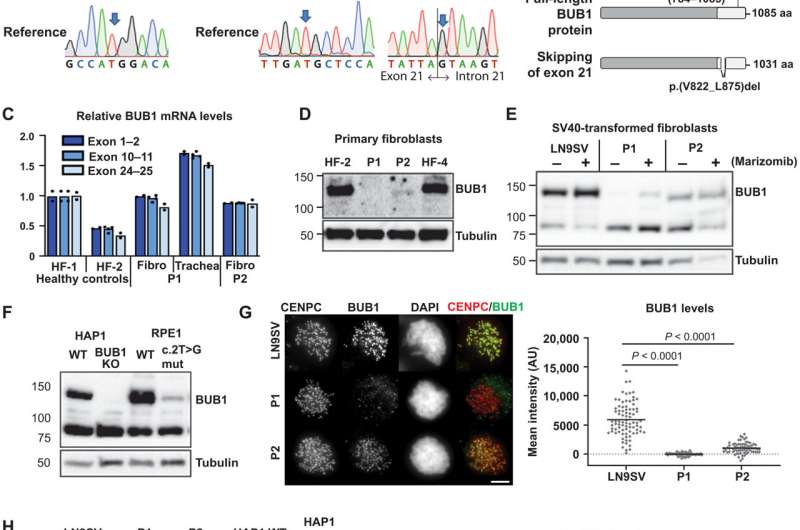
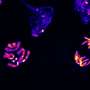
FIG. 1. Biallelic germline mutations in BUB1 cause reduced protein levels.(A) Family pedigrees (based on WES) and Sanger confirmation from primary fibroblasts. Nomenclature NM_004336.5. (B) P2 primary fibroblast cDNA was PCR-amplified using indicated primers (red arrows) and analyzed on agarose gel and by Sanger sequencing. The transcript lacks exon 21. Predicted effect on BUB1 protein is shown. (C) BUB1 mRNA was assessed in indicated cells using three different primer pairs, each in three technical replicates. (D and E) Western blot of primary fibroblasts (D) (using A300-386A to detect BUB1) and SV40-transformed fibroblasts (E) (using ab9000). CDC6 is a control for marizomib-induced protein stabilization (6 hours, 500 nM). (F) Western blot of engineered RPE1-BUB1 mutant cells and complete BUB1 KO HAP1 cells using ab9000. (G) Colchicine-arrested cells (10 μM, 2 hours) were stained for CENPC, BUB1 (using A300-386A), and DNA. Graph depicts mean BUB1 intensity within CENPC-defined regions. n = 83, 73, and 72 cells from three independent experiments. (H) Colchicine-arrested cells, treated with BUB1 kinase inhibitor BAY1816032 (4 μM, 1 hour) or dimethyl sulfoxide (DMSO), were stained, and mean pH2A-T120 intensity was assessed within DAPI-defined regions. Approximately 90 cells from three independent experiments were analyzed per condition. Each dot represents one cell; black lines indicate the means. Scale bars, 5 μm. Nonparametric Kruskal-Wallis test (Dunn’s multiple comparison test) used for statistical analysis. AU, arbitrary units; aa, amino acid. Credit: DOI: 10.1126/sciadv.abk0114
When cells divide, through a process called mitosis, the genetic information must be equally distributed among the new cells. That is the moment when mitotic errors can arise, potentially leading to DNA damage, including damage to the chromosomes or alterations in their number. These errors are associated with several human pathologies.
One of the proteins responsible for monitoring problems in cell division is the one coded by the BUB1 gene. Defects in BUB1 have been associated with cancer, however, its role in congenital human development has remained unclear. Low expression of BUB1 has been associated with spontaneous miscarriages and given its importance for correct cell division, deleterious mutations in this gene were, until now, thought to be incompatible with life.
A collaborative effort involving clinicians and researchers from Portugal, Austria, and the Netherlands has led to the identification of the first known patients with BUB1 biallelic mutations (in both copies). "It was surprising to find patients with mutations in such a critical gene," explains Raquel Oliveira, leader of the team from the Instituto Gulbenkian de Ciência (IGC) who conducted the study. Still, both patients displayed microcephaly (a congenital malformation in which the brain is smaller than average), intellectual disability and several patient-specific features.
Researchers at the IGC explored how the pathological variants of this gene affected cell division. "We showed how each mutation affected protein abundance and function, and their impact on various pathways that control the fidelity of mitosis," says Sara Carvalhal, first author of the study and former IGC researcher. Although both patients presented cells with abnormal chromosome structure or numbers, the molecular mechanisms underlying these errors in cell division were different between them. According to Job de Lange, researcher from Cancer Center Amsterdam who co-lead the work, "these findings enable the dissection of the different roles of BUB1 in a clinical context."
Besides providing new insight on the roles of BUB1 , this study also sheds light on "how mitotic defects impact the development of the human body," explained Sara. These findings support the idea that the developing brain is particularly susceptible to problems in mitosis. The authors suggest that the described mitotic errors might cause the death of cells that are important for brain development, accounting for the clinical manifestations of this novel inherited neurodevelopmental disorder, namely microcephaly and intellectual disability. Sara Carvalhal will now be pursuing this line of research and studying the effects of cell division errors on rare diseases at her own laboratory at the Algarve Biomedical Center.
Most rare diseases are understudied and often difficult to diagnose. In the future, these new findings might prevent the undefined or misdiagnosis of patients with deleterious BUB1 mutations, with direct consequences for their treatment, prognosis, and follow-up. "In children with primary microcephaly and multiple congenital malformations biallelic germline-mutations in BUB1 should be considered as causal more often than it was done until now," says Ingrid Bader, MD and co-author of the study, from Paracelsus Medical University, Salzburg, Austria, currently at Institute for Medical Genetics and Applied Genomics in Tuebingen, Germany. This research also contributes to a better understanding of rare syndromes that clinical or molecularly overlap with the patients here described, including mosaic variegated aneuploidy (MVA), cohesinopathies and primary microcephaly (MCPH).
More information: Sara Carvalhal et al, Biallelic BUB1 mutations cause microcephaly, developmental delay, and variable effects on cohesion and chromosome segregation, Science Advances (2022). DOI: 10.1126/sciadv.abk0114
Journal information: Science Advances
Provided by Instituto Gulbenkian de Ciência (IGC)