Study: Post-surgical monoclonal antibody treatment reduces breast cancer recurrence
Treating women diagnosed with one type of early-stage breast cancer with the monoclonal antibody trastuzumab after surgery reduces the risk of the cancer returning, reports a research team led by University of Saskatchewan (USask) oncologist Dr. Shahid Ahmed (MD).
The team's study, published Jan. 20 in Scientific Reports, examined the medical records of all Saskatchewan women diagnosed with a small HER2-positive breast cancer between January 2008 and December 2017. Among the most aggressive of breast cancers, HER2 is named for the protein human epidermal growth factor receptor 2 that malfunctions to cause rapid cancer cell growth.
"HER2-positive breast cancer has been associated with high risk of recurrence," said Ahmed, a professor and division head of oncology at USask's College of Medicine.
A 12-month course of treatment with trastuzumab in conjunction with chemotherapy after surgery is the standard treatment for early-stage HER2-positive breast cancer tumors larger than a centimeter or have spread to lymph nodes, Ahmed said.
However, the benefit was not well-known of adjuvant (post-operative) trastuzumab treatment for smaller HER2-positive tumors that haven't spread beyond the initial site (metastasized) to lymph nodes.
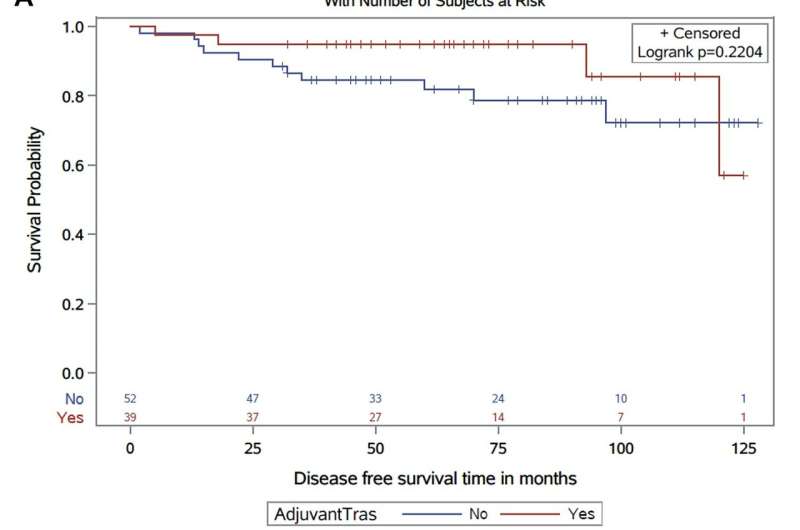
Using the health records, Ahmed's team identified 91 women who had early-stage, non-metastasized HER2-positive breast cancers smaller than 10 millimeters. Overall, 39 patients whose tumors mostly were larger than 5mm had received adjuvant trastuzumab and chemotherapy, while 52 with smaller tumors had not received post-operative trastuzumab.
While the sample size is small, the study is population-based with no selection bias and covers a 10-year period, Ahmed said.
"Our study showed that women with HER2 positive tumors less than one centimeter and were node-negative had a low rate of recurrence," Ahmed said. "However, those who received adjuvant trastuzumab had a further reduction in the risk recurrence. For example, women who did not receive adjuvant trastuzumab had a four-fold greater risk of recurrence."
Overall, 97 percent of women in the trastuzumab group were free of a breast cancer recurrence after 10 years, compared to 88 percent among those who didn't receive the treatment.
The analysis of this retrospective cohort study was statistically adjusted to account for such factors as patient ages and other risks, to make the findings as close as possible to those of a randomized study, Ahmed said.
He anticipates the findings of the study, which favor adjuvant trastuzumab for small tumors, will lead to its more frequent use to reduce the risk of cancer recurrence in younger women with tumors larger than 5mm.
However, since treatment toxicity is an important consideration in contemplating the use of adjuvant trastuzumab, a key point to examine is whether the duration of treatment should be shortened to six months from the current 12 months, he said.
More information: Sanji Ali et al, Efficacy of adjuvant trastuzumab in women with HER2-positive T1a or bN0M0 breast cancer: a population-based cohort study, Scientific Reports (2022). DOI: 10.1038/s41598-022-05209-8

















