Using stem cells to regenerate the heart
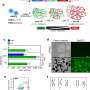
![Fig. 1: Defined surfaceome transcript of the developing epicardium revealed the enrichment of CD22 and CDH18 in late-stage populations. a PCA using the GSE84085 RNA-Seq expression dataset of d12 early epicardial-like cells (Epi12, red) (19-9-7-Epi, n = 2), d48 late epicardial-like cells (Epi48, brown) (H9-Epi, ES03-Epi and 19-9-11-Epi, n = 6) and human adult epicardium (dark violet) (donors 9605, 9633, 9634 and 9635, n = 8). b Venn-Diagram showing genes in significant absolute correlation to the epicardial markers WT1 (light green), TBX18 (light pink) and ALDH1A2 (light purple) (Pearson correlations; R ≥ 0.6) as well as cell surface marker enrichment (target). c Overlap of the target gene set from b (gray) against DEGs (Supplementary Fig. 1e) (light brown) for the surfaceome transcript identification of the epicardium. d Heatmap showing correlations (Pearson, R, 1 to −0.7, color scale) of surface marker expression relative to the expression of endothelial cell markers CDH5 and PECAM1. e Fold change of surface marker expression in Epi48 (brown) relative to Epi12 (red). f Heatmap showing correlations (Pearson, R, 0.6–1, color scale) of the top three genes from (e) to epicardial markers. g Retrospective analysis showing a heatmap depicting active epicardial markers and CD22 and CDH18 expression in embryonic and adult cells. h Analysis for CD22 and CDH18 expression in CF (left) and SMC (right) with representative CF genes (yellow) and SMC genes (light blue) [error bars indicate standard derivation (SD)]. i Heatmap showing correlations (Pearson, R, 0 to −1, color scale) of CD22 and CDH18 for CF markers PDGFRA, FAP and POSTN and SMC markers CNN1, ACTA2, and TAGLN. j GSE106168 retrospective analysis for CDH18 expression in different cardiac cellular clusters [(C1—5W whole heart (n = 257); C2—Cardiomyocytes (n = 1492); C3—fibroblast-like cells (n = 786); C4—endothelial cells (n = 445); C5—heart valves (n = 427); C6—epicardial -EPI- cells (n = 46); C7—immune cells (n = 27); C8—macrophages (n = 308); C9—T and B lymphocytes (n = 58)] k GSE106168 retrospective analysis for CDH18 expression in epicardial-EPI-cluster [UPK3Bhigh WT1high TBX18high ALDH1A2high] ranging different time points of gestation [5 weeks, n = 30; 22 weeks, n = 46]. Credit: DOI: 10.1038/s41536-022-00207-w Using stem cells to regenerate the heart](https://scx1.b-cdn.net/csz/news/800a/2022/using-stem-cells-to-re.jpg)
Heart disease remains the leading cause of death in the world. One reason is that unlike other tissues, such as bone and skin, the heart has remarkably poor regenerative capability after an injury such as a heart attack. Scientists have therefore searched for heart cells that have regenerative properties. A new study by the Yoshinori Yoshida laboratory reports the use of iPS cells to produce one such cell type, epicardial cells.
The epicardium describes the most outer layer of the heart. As the heart forms, epicardial cells migrate and differentiate into different heart cells. However, after birth, the epicardium takes a quiescent state and only reactivates following injury to produce fibrosis and scarring. While this response is not ideal and fails to fully recover heart function, in some species, such as zebrafish, epicardium reactivation is a critical step for heart regeneration.
In general, the epicardium of a growing fetal heart behaves differently from that of a fully formed adult heart.
"The embryonic epicardium is active. Its cells are proliferative and undergo an epithelial-to-mesenchymal transition that results in various cardiac cell types. This property is not seen in adult heart," said Julia Junghof, who is the first author of the study and is expected to earn her Ph.D.
To study these differences, she and colleagues differentiated human iPS cells into epicardial cells. They found the protein cadherin 18 (CDH18) was expressed in only the fetal type.
"We normally rely on the expression of genes to identify epicardial cells but do not have reliable surface markers. Cadherins are best known for cell-to-cell adhesion, but they also are involved in cell-fate decisions," said CiRA Associate Professor Yoshinori Yoshida, who led the study.
Artificially expressing genes in iPS cells is technically difficult and expensive. Using surface markers reduces the labor and cost of the experiments and also results in safer cells that can be used later for patient care.
The study shows cadherin 18 (CDH18) is such a surface marker. CDH18 is better known for its role in axon development and maintenance in the nervous system, but the study showed its usefulness for identifying iPS cells that successfully differentiated into epicardial cells. Notably, while CDH18 was expressed in fetal epicardial cells, it was not expressed in adult epicardial cells, which could reflect the different regenerative properties of the heart.
The loss of CDH18 expression resulted in epicardial cells losing their ability to multiply and caused an epicardial-to-mesenchymal transition that resulted in smooth muscle cells for the heart, providing new insights on how the epicardium can be activated following injury to promote heart regeneration.
Thus, measuring the expression of CDH18 on differentiating iPS cells may provide an effective means for producing cells with heart regeneration ability in the lab.
CiRA scientist Dr. Antonio Lucena-Cacace, a senior author of the study, added that the findings also give clues on how heart regeneration can be turned on and off in the body.
"Our findings show how regulation of the epicardial-to-mesenchymal transition affects the cell fate and regenerative responses of epicardial cells," he said.
More information: Julia Junghof et al, CDH18 is a fetal epicardial biomarker regulating differentiation towards vascular smooth muscle cells, npj Regenerative Medicine (2022). DOI: 10.1038/s41536-022-00207-w