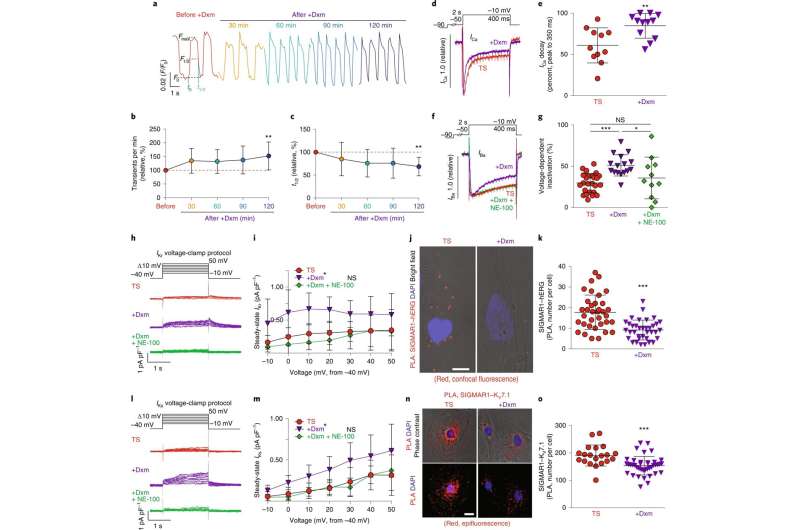
The effect of dextromethorphan on cardiac Ca2+ handling and ion channels in a human iPSC model of TS. a, Representative traces of time-course Ca2+ imaging in spontaneously contracting cardiomyocytes from patients with TS treated with dextromethorphan (5 μM, until 120 min). b,c, Ca2+ transient frequency (b) and duration (c) analyses of cardiomyocytes from patients with TS before and after dextromethorphan treatment (n = 19). d, Representative traces of Ca2+ currents in cardiomyocytes from patients with TS with and without dextromethorphan. e, Late Ca2+ current analysis of cardiomyocytes from patients with TS with and without dextromethorphan treatment (TS, n = 11; with dextromethorphan, n = 12). f, Representative traces of Ba2+ currents in cardiomyocytes from patients with TS without treatment or treated with dextromethorphan (5 μM, 2 h, dextromethorphan) or with dextromethorphan and a SIGMAR1 antagonist, NE-100 (1 μM, dextromethorphan and NE-100). g, Voltage-dependent inactivation in cardiomyocytes from patients with TS without treatment (n = 25) or treated with dextromethorphan (n = 16) or with dextromethorphan and NE-100 (n = 11). h, Representative traces of IKr currents (E-4031 sensitive) in cardiomyocytes from patients with TS treated with dextromethorphan (5 μM) or dextromethorphan and NE-100 (each at 5 μM) or without treatment. i, IKr current amplitude analysis of cardiomyocytes from patients with TS treated with dextromethorphan (n = 9) or dextromethorphan and NE-100 (n = 10) or without treatment (n = 10) (*P < 0.05, dextromethorphan versus TS and dextromethorphan versus dextromethorphan and NE-100 at −10, 0, 10, 20 and 30 mV). j, Representative confocal fluorescent and bright-field images of cardiomyocytes from patients with TS without and with dextromethorphan treatment from the PLA (SIGMAR1–hERG, red; 4,6-diamidino-2-phenylindole (DAPI), blue). Scale bar, 10 μm. k, Quantification of SIGMAR1–hERG by the PLA in dextromethorphan-treated (n = 36) and untreated (n = 35) cardiomyocytes from patients with TS. l, Representative traces of IKs currents (chromanol 293B sensitive) in cardiomyocytes from patients with TS treated with dextromethorphan (5 μM) or dextromethorphan and NE-100 (each at 5 μM) or without treatment. m, IKs current amplitude analysis of cardiomyocytes from patients with TS treated with dextromethorphan (n = 10) or dextromethorphan and NE-100 (n = 9) or without treatment (n = 10) (*P < 0.05, dextromethorphan versus TS and dextromethorphan versus dextromethorphan and NE-100 at −10, 0, 10, 20, 30 and 40 mV). n, Representative epifluorescent and phase-contrast images of cardiomyocytes from patients with TS without and with dextromethorphan treatment from the PLA (SIGMAR1–KV7.1, red; DAPI, blue). Scale bar, 10 μm. o, Quantification of SIGMAR1–KV7.1 by the PLA in dextromethorphan-treated (n = 40) and untreated (n = 20) cardiomyocytes from patients with TS. All data are mean ± s.d. One-way ANOVA with Tukey’s multiple comparisons was used for b,c,g between groups and for i,m at each voltage step. Unpaired two-tailed Student’s t-tests were used for e,k,o. *P < 0.05, **P < 0.01, ***P < 0.001. Samples were from at least two independent differentiations. Credit: DOI: 10.1038/s44161-021-00016-2
An over-the-counter cough suppressant can knock some heart cells back into rhythm, a finding that may lead to a new way to treat a rare heart condition called long QT syndrome.
The discovery was made with the help of stem cells from patients with the disorder.
In people with long QT syndrome, heart cells are not always ready to produce the next beat, a situation that can knock the heart out of its normal rhythm, which may be life-threatening. For many people with long QT, no treatment can correct the heart cells or prevent arrythmia.
Understanding how human heart arrythmias can be stopped is difficult to study with mice, so Columbia's Masayuki Yazawa, Ph.D., turned to patient-derived reprogrammed stem cells, which can be made into heart cells in the lab.
Heart cells in a dish
The cough suppressant discovery began several years ago when Yazawa found that heart cells in the lab would resume a normal rhythm when a certain enzyme was inhibited. But the drugs used to inhibit the enzyme also had other unintended effects, such as liver toxicity.
"We had to find alternatives," says Yazawa, assistant professor of rehabilitation & regenerative medicine at the Columbia University Vagelos College of Physicians and Surgeons and also a core faculty member of Columbia Stem Cell Initiative.
Yazawa's team reviewed published studies for ideas and learned that the enzyme could be inhibited through an intermediary molecule inside heart cells called SIGMAR1. Further reading suggested that SIGMAR1 could be targeted by a cough suppressant, dextromethorphan.
Heart cells on cough medicine
In the new study, Yazawa's team found that the cough suppressant, when added to heart cells, successfully prepared the heart cells for the next beat and soothed the cells' irregular rhythm.
The cough suppressant reset heart cells from people with Timothy syndrome, a genetic disorder that also causes other heart abnormalities, and from people with more common forms of long QT syndrome.
Yazawa cautions that it's premature to use dextromethorphan to treat long QT patients; the drug has a short half-life and would have to be used long term, which might still have unknown adverse side effects.
"But our study shows that drugs targeting SIGMAR1 have potential to treat a wide array of patients with long QT syndrome," says Yazawa, "and we will continue to look for better options."
Says Emmanuelle Passegué, Ph.D., Alumni Professor of Genetics and Development and director of the Columbia Stem Cell Initiative: "This is a beautiful example of how patient-derived reprogrammed stem cells (or induced pluripotent stem cells) can be used to discover new treatments for uncurable conditions and improve patient health."
The paper, "Sigma non-opioid receptor 1 is a potential therapeutic target for long QT syndrome," was published Feb. 17 in Nature Cardiovascular Research.
More information: LouJin Song et al, Sigma non-opioid receptor 1 is a potential therapeutic target for long QT syndrome, Nature Cardiovascular Research (2022). DOI: 10.1038/s44161-021-00016-2
Provided by Columbia University