Credit: Pixabay/CC0 Public Domain
With as much turmoil and negativity as the COVID-19 pandemic has supplied, there may be a silver lining when it comes to a new vaccine study for those suffering with HIV.
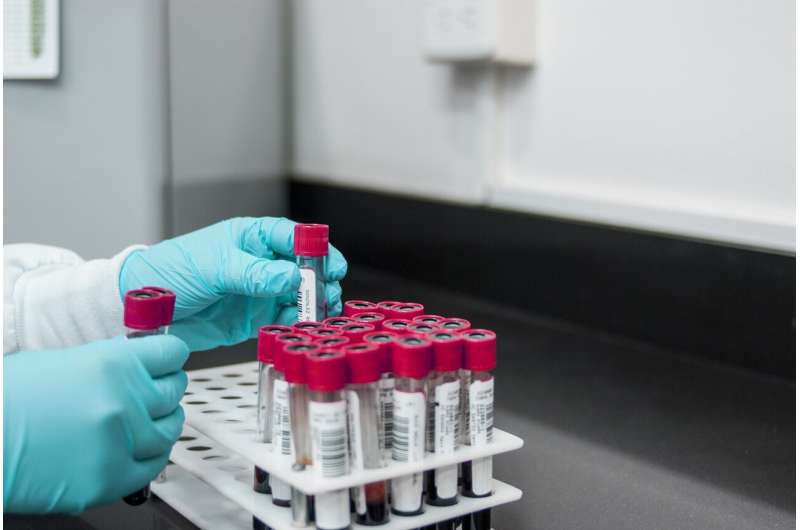
The first study participant has been enrolled in a new Phase 1 clinical trial using the messenger ribonucleic acid, or mRNA, vaccine technology developed by Moderna to evaluate the safety and immune responses of three different experimental vaccines against HIV. This randomized, open-label trial represents one of the first clinical studies of the use of mRNA vaccine technology against HIV.
The University of Alabama at Birmingham enrolled the trial's second participant and has enrolled several others since.
Paul Goepfert, M.D., director of the Alabama Vaccine Research Clinic in the Marnix E. Heersink School of Medicine, says the emergence of mRNA vaccines to combat COVID-19 certainly helped move this mRNA HIV vaccine trial along.
"Despite tremendous advancements in treatment and prevention of HIV, we still do not have an effective vaccine and thousands of Americans continue to be infected every year," Goepfert said. "mRNA technology enables a much more rapid development of vaccines that was not previously possible. This new technology will certainly decrease the time it will take to develop an effective HIV vaccine."
The study, HVTN 302, will enroll up to 108 HIV-negative adults. The primary study hypothesis is that the mRNA vaccines will be safe and well-tolerated among HIV-negative people and will elicit neutralizing antibodies.
The experimental vaccines carry mRNA, a piece of genetic code, delivered with instructions for making proteins in the same way that the mRNA vaccines against COVID-19 instruct the body's cells to make the SARS-CoV-2 spike protein. These instructions show human muscle cells how to make small portions of proteins that resemble parts of HIV but are not the actual virus. People cannot contract HIV from the vaccines. Once immune cells have used the instructions, the mRNA is quickly broken down, and does not stay in the body.
The investigational vaccines are not expected to provide protection from HIV infection, yet the knowledge gained from this study will aid in the future development of an HIV vaccine regimen. Researchers hope to learn whether the immune system will respond to the experimental vaccines by making antibodies and T cells that could fight HIV if a person is ever exposed to the virus in the future. The trial will also clarify whether the immune response to an mRNA vaccine is equal to or better than the response to a protein-based vaccine, while helping define the potentials of using mRNA to increase the pace of developing an HIV vaccine.
Provided by University of Alabama at Birmingham