Study shows more women with invasive lobular breast cancer should qualify for tailored clinical trials
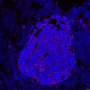
![Summary of mutational analysis. (A) Bar plot of number of SNVs for each component of patient samples. (B) Bar plot of indels for each component of patient samples. (C) Proportion of SNVs split into six classes in pyrimidines context. (D) Heatmap of recurrently altered breast cancer genes [6] with at least one mutation found in our study. Numbers on bar plots show overlap coefficient of SNVs (A) and indels (B) between paired patient samples. Note that ML6_Abr has a ploidy estimate of 4, so is copy neutral with LOH of the CDH1 locus. (E) MOBSTER-inferred clonal clusters of ML8_Neg showing two distinct VAF-derived distributions. Credit: Cancers (2022). DOI: 10.3390/cancers14020295 Study shows more women with invasive lobular breast cancer should qualify for tailored clinical trials](https://scx1.b-cdn.net/csz/news/800a/2022/study-shows-more-women-1.jpg)
A new study led by scientists at The Institute of Cancer Research, London, has changed our understanding of invasive lobular breast cancer—and could increase the number of patients who access new treatments via lobular breast cancer specific clinical trials.
In the study, researchers showed that the diagnosis criteria for invasive lobular breast cancer—a type accounting for 10–15% of breast cancer cases—should be expanded to include an extra subset of tumors that have not previously been included.
The study, which was published in a special issue of Cancers and funded by Breast Cancer Now, could impact the diagnosis of up to 10% of patients with invasive lobular breast cancer, who currently may miss out on the chance to access clinical trials for new potential treatments.
Diagnosis criteria
Invasive lobular breast cancer, which develops in the lobes of the breast that produce milk, is usually diagnosed by the loss of a protein called E-cadherin. The loss of E-cadherin, which is involved in helping cells stick together, can be seen under a microscope in tumor samples that have been stained with a special dye.
In some patients, however, this protein is only lost in parts of the tumor, while other parts still contain it or show abnormal E-cadherin staining, making a definitive diagnosis of lobular breast cancer difficult.
Comprehensive DNA analysis supports expansion of diagnosis criteria
A team of researchers at the ICR sought to address whether these mixed tumors were similar enough genetically to the E-cadherin deficient tumors to be classed as the same type.
They used whole genome sequencing and methylation analysis—a measure of DNA activation—on two samples of the same tumor from nine individual patients: one from an area completely lacking E-cadherin and one where E-cadherin was still appearing on the surface of, or misplaced inside, cells.
The researchers revealed that the two samples from each patient were very similar in both their mutations and methylation profiles. Importantly, most "heterogenous," or mixed, tumors also harbored mutations to the E-cadherin gene in both the apparently E-cadherin positive and negative areas.
Taken together, these findings suggest that tumor cells from both locations arose from common ancestor cancer cells in each patient, and both should be classified as invasive lobular breast cancers.
Broadening access to clinical trials
Study co-leader Dr. Rachael Natrajan, team leader of the Functional Genomics Team in the Breast Cancer Now Toby Robins Research Centre at the ICR, said: "Our study provides the first comprehensive genome-wide characterization of invasive lobular breast cancers with varied E-cadherin expression and shows they should be considered as being similar to more classical E-cadherin deficient tumors. An important implication of this work is that women with these atypical tumors could still benefit from new therapies for invasive lobular breast cancer and should be offered the chance to take part in the same clinical trials."
The phase II ROLo trial, for example, which is based on earlier work by Professor Chris Lord and his team at the ICR and was funded by Breast Cancer Now, excludes women whose tumors show partial or abnormal E-cadherin staining. The work from Dr. Natrajan's team suggests that these women should also be included in such trials.
Study co-leader, Dr. Syed Haider, leader of the Bioinformatics Group in the Breast Cancer Now Toby Robins Research Centre at the ICR, said: "While whole genome sequencing studies are rapidly becoming more common, there remains an unmet need for detailed characterization of tumors with specific molecular traits in rare subtypes of breast cancer. Here, we used a cost-effective technology to study the whole genomes of mixed invasive lobular breast cancers, showcasing the remarkable potential of big data in taking us a step closer to delivering precision medicine."
More information: John Alexander et al, Assessment of the Molecular Heterogeneity of E-Cadherin Expression in Invasive Lobular Breast Cancer, Cancers (2022). DOI: 10.3390/cancers14020295