Second-generation drug-coated balloon superior for opening blocked arteries in the leg
Patients treated for a blocked artery in the leg were significantly more likely to have an open artery without further interventions at 12 months if they received the second-generation paclitaxel-coated balloon catheter known as the Chocolate Touch device compared with those receiving the commercially-available Lutonix drug-coated balloon catheter, according to a study presented at the American College of Cardiology's 71st Annual Scientific Session.
The trial met its primary efficacy endpoint for non-inferiority and showed an improvement in terms of the proportion of patients with adequate blood flow through the artery without subsequent procedures. The trial also found the Chocolate Touch device was non-inferior to the Lutonix device in terms of safety.
"I'm very excited that we now have a head-to-head comparison of a second-generation vs. first-generation drug-coated balloon and data that we can rely on to make decisions for our patients," said Mehdi Shishehbor, DO, MPH, Ph.D., president of the Harrington Heart and Vascular Institute at University Hospitals of Cleveland and the study's lead author. "I think that the superiority of Chocolate Touch indicates that as we improve the technologies, there is additional benefit to be gained. For me, this study makes the case that this [Chocolate Touch] will be the device of choice between these two devices for patients who require drug-coated balloon therapy."
The trial enrolled 313 randomized patients treated for superficial femoral and popliteal artery disease—conditions that involve blocked arteries in the upper leg—at 34 sites in four countries. All patients underwent balloon angioplasty, a procedure to reopen the artery by threading a tiny device into the blocked area and inflating a small balloon covered in medicine that helps prevent blockage from reoccurring. In half of the patients, operators used the Lutonix drug-coated balloon, and the other half were treated with a Chocolate Touch balloon. The Chocolate Touch device is designed with a structure that constrains the balloon, creating pillows and grooves that are reminiscent of the shape of a chocolate bar.
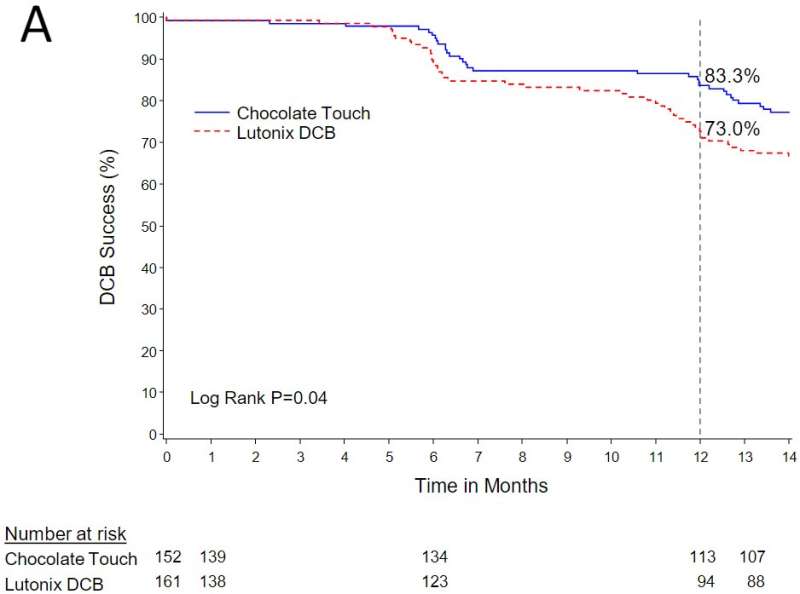
At 12 months, 78.8% of the patients who received the Chocolate Touch device and 67.7% of those receiving the Lutonix device achieved the primary endpoint of patency, or blood flow as measured by the peak systolic velocity ratio, a significant difference demonstrating the superiority of the Chocolate Touch device. In terms of safety, the trial found no statistical difference between Chocolate Touch and Lutonix, with a rate of major adverse events of 11.1% in the Chocolate Touch arm and 15.4% in the Lutonix arm. Major adverse events included a composite of death related to the targeted limb, major amputation and revascularization procedures.
Shishehbor said that estimates of mortality on a cumulative, year-by-year basis are consistently lower in those patients treated with Chocolate Touch as compared to those receiving Lutonix. For patients who have reached the three-year follow-up, estimated mortality is 6.8% among patients receiving the Chocolate Touch device, which was also well below the trial's prespecified goal of 13.2%. This finding offers reassurance that the paclitaxel coating used in the device had a favorable safety profile.
"At a minimum, this [Chocolate Touch] device is as safe as the Lutonix, with a trend for lower mortality rates," Shishehbor said.
The Chocolate Touch device is designed to provide a more even and controlled widening of the artery. This design allows operators to use a slightly larger balloon to open the artery wider and provide increased contact between the balloon surface with the paclitaxel coating, Shishehbor said.
Although balloon angioplasty is the preferred treatment for blocked arteries in the leg, many patients experience recurring blockages, requiring additional procedures.
"If we are able to offer patients therapies that can keep the artery open for as long as possible, that will be welcome news," Shishehbor said. "As we advance our technologies and get more patency, or blood flow, over time, the patients will enjoy that benefit and have a lower likelihood of needing repeat procedures."
Shishehbor said that the trial paused enrollment for six months in response to industry-wide concerns over the safety of paclitaxel but said that the study ultimately achieved a high follow-up rate of 94%. The trial is also the first to allow operators to combine the drug-coated balloon treatment with atherectomy, in which a blade is used to first remove plaque from the vessel prior to drug-coated balloon use. Shishehbor said that while only a small number of patients was treated with atherectomy, the patency rate was promising. Future studies could determine whether the Chocolate Touch device may have an even greater advantage when used to treat longer and more complex blockages.
This study was simultaneously published online in Circulation at the time of presentation.
More information: Mehdi H. Shishehbor et al, A Randomized Trial of Chocolate Touch Compared with Lutonix Drug-Coated Balloon in Femoropopliteal Lesions (The CHOCOLATE TOUCH Study), Circulation (2022). DOI: 10.1161/CIRCULATIONAHA.122.059646