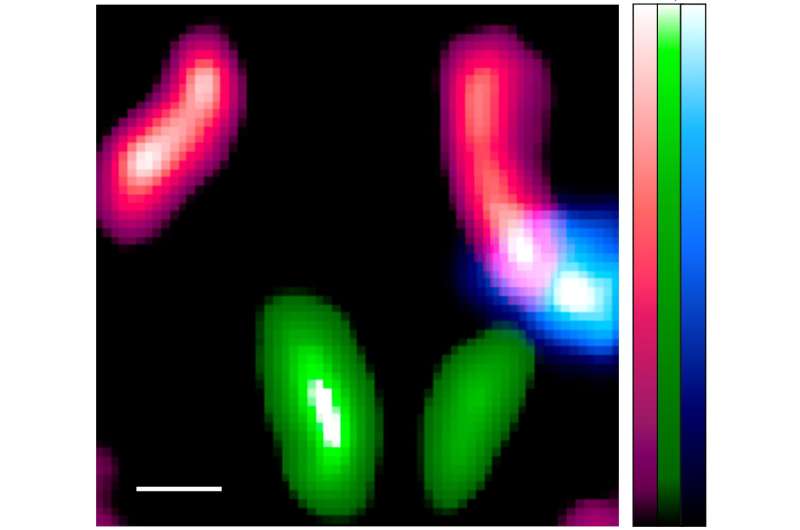
The image shows three nuclides are clearly identifiable and separated. Our imaging system has made it possible to identify and separate each nuclide and produce images such as those shown in Figure 4c.The red shows the Indium-111 in the lymph nodes below the mouse jaw, green shows the Iodine-125 in the thyroid, and the blue shows the technetium-99m in the lymph nodes below the ear. The white line at the bottom left of the image shows the scale of the image: 1mm. Credit: Yagishita et al.
Researchers have developed a biomedical imaging system using a semiconductor detector originally developed for hard X-ray and gamma-ray space observation, and used a spectral analysis method from astronomy to take accurate images of multiple radionuclides in small animals, reports a new paper in Nature Biomedical Engineering published on April 4.
Currently, fluorescent tracers are used to image the distribution of multiple molecules in a sample under a microscope. The use of multiple tracers can reveal the distribution of numerous molecules in detail. However, when it comes to imaging molecules within the body of animals, including humans, this becomes difficult as the body tissue absorbs most of the light. So, researchers use radionuclides as probes since radiation is absorbed much less by animal tissues than optical light, allowing them to obtain images of the distribution of tracers in the body.
However, current technology makes it somewhat difficult to distinguish between two or more nuclides, and has low spatial resolution. Also, it is difficult to remove noise on the image caused by radiation from other sources.
To try and resolve this problem, a collaboration of researchers from fields who usually never meet one another, led by Project Assistant Professor Atsushi Yagishita from the Kavli Institute for the Physics and Mathematics of the Universe (Kavli IPMU), RIKEN, the Institute of Space and Astronautical Science, and the National Cancer Center Japan, started a project in 2018 to adapt technology used in space observation for medical research.
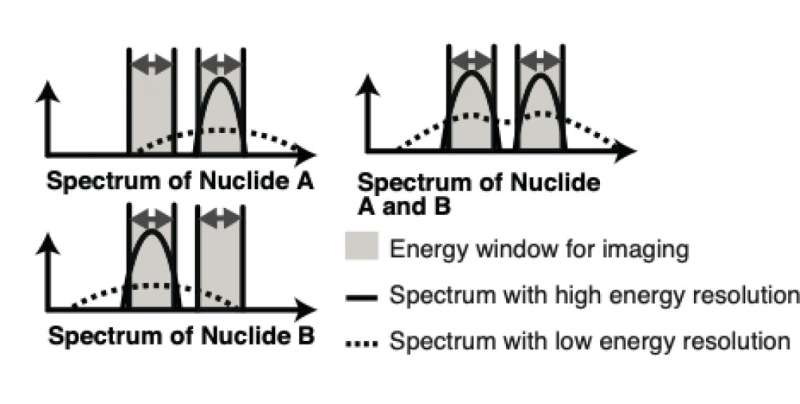
Figure 1: Model explaining the energy resolution. The top left image shows the spectrum of nuclide A, and the bottom left image shows the spectrum of nuclide B. When nuclide A and B are both present, the spectrum looks like the top right image. Imagers with limited energy resolution detect nuclides A and B as a low plateau with two small peaks, but an imaginer with improved energy resolution can detect individual A and B spectra. Credit: Yagishita et al.
Their latest study highlights two achievements.
One of the team's achievements was the development of an imager, named IPMU imager, which was equipped with a cadmium telluride semiconductor detector with high energy resolution, meaning it had the ability to distinguish radiations of different energies, and a multi-pinhole collimator that could achieve high spatial resolution for radionuclide imaging. The IPMU imager made it easy to distinguish between radiations with different energies.
Still, there was room for improvement. The IPMU Imager could not eliminate all noise, especially when two radionuclides released similar radioactive emissions.
So the team's second achievement was to use "fitting," a spectroscopic X-ray analysis method used in the field of astronomy. What they were able to find was that using the technique allowed them to identify the source of all radiation. This eliminated noise radiation and made it possible to obtain accurate images using only radiation from the target nuclide.
-
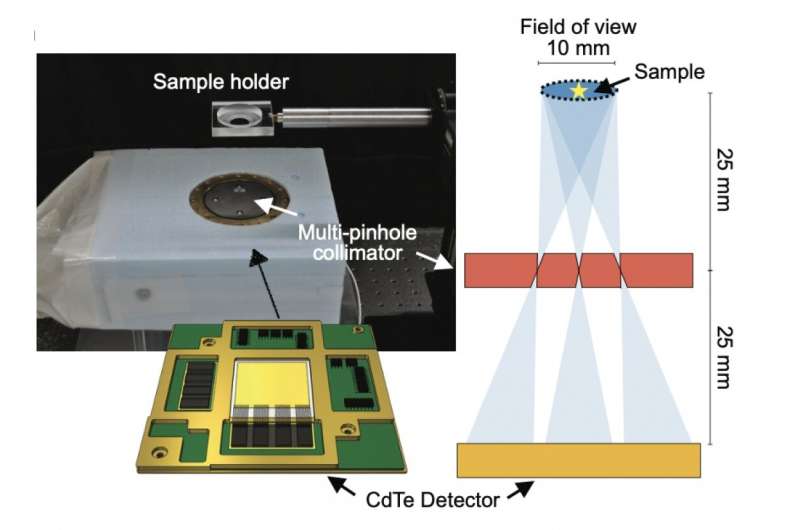
Figure 2. The imaging device and illustration of the setup. The cadmium-telluride semiconductor detector is installed inside the multi-pinhole collimator box. Credit: Yagishita et al./Kavli IPMU
-
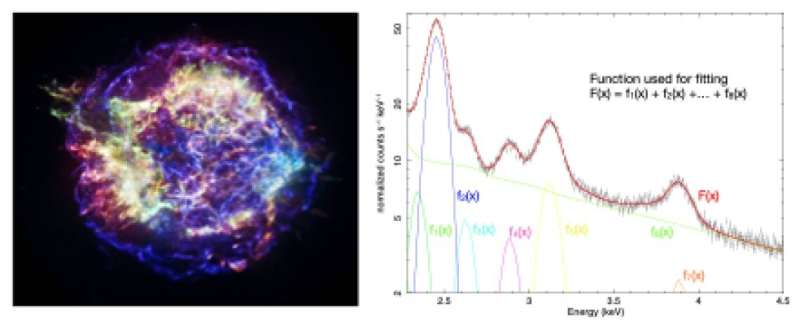
Figure 3. Conceptual diagram showing the spectral fitting technique for observational data of supernova remnant Cassiopeia A. The right panel shows an analyzed X-ray energy spectrum (note: this is a simplified version of an actual analysis process, carried out to provide a background explanation to this study). In a proper analysis, the physical aspects of the X-ray energy spectrum are considered, and the spectral analysis is performed to identify the elemental distribution of the supernova remnant, etc. Credit: Left image: NASA/CXC/SAO. Right image: Kavli IPMU
-
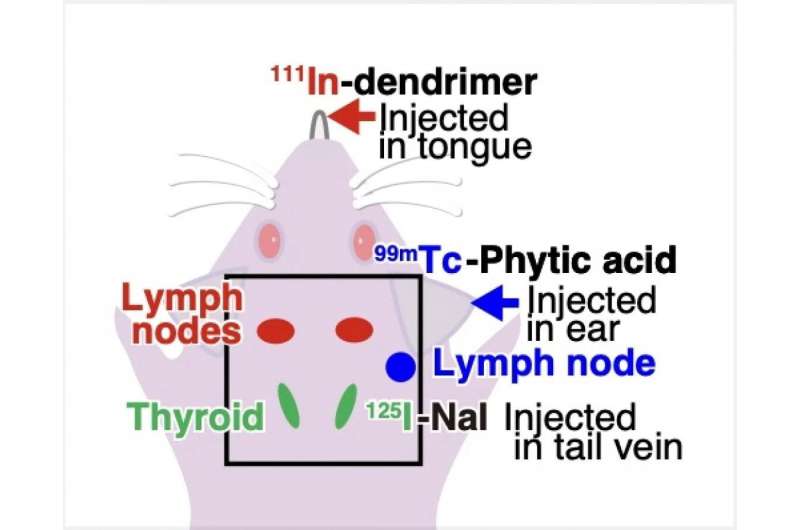
Figure 4a. The diagram shows where the three types of tracers accumulate in the mouse. Technetium-99m in the lymph node under the ear, indium-111 in the lymph nodes below the jaw, and iodine-125 in the thyroid. Credit: Yagishita et al./Kavli IPMU
-
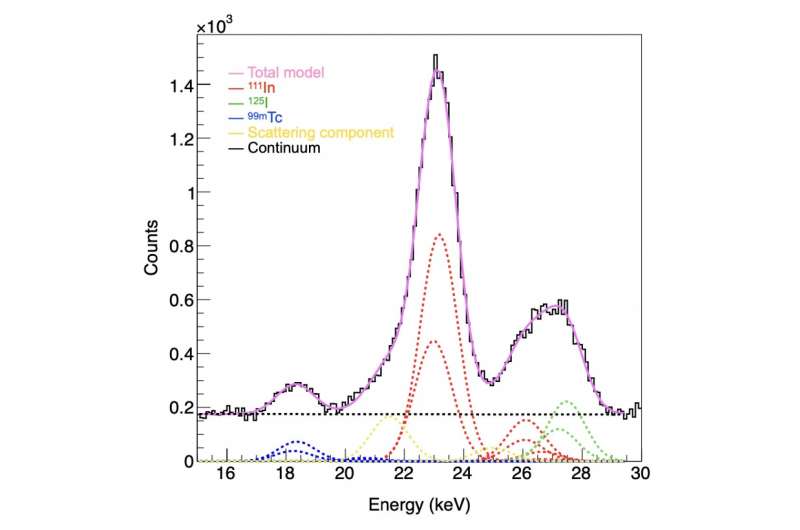
Figure 4b: The total spectrum (black solid line) and the individual spectra (colored dotted lines) identified and separated using the fitting method. The black line indicates the data obtained by the imager. Indium-111 (red), iodine-125 (green), and technetium-99m (blue) spectra were separated from the overall spectrum by fitting. Credit: Yagishita et al./Kavli IPMU
-
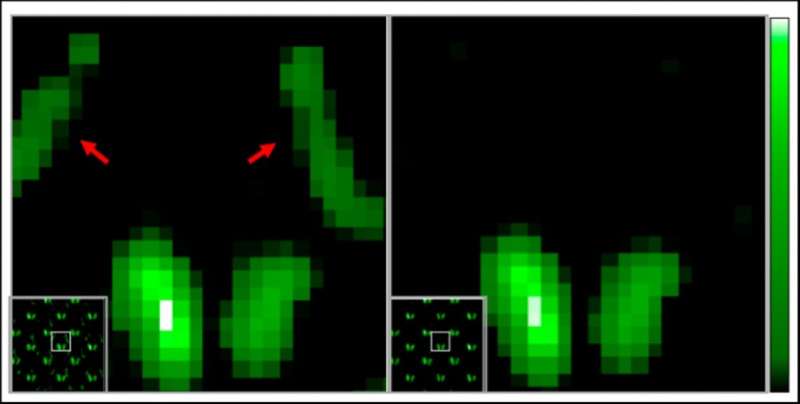
Figure 4c: A representative mouse image in iodine-125 energy window (26 – 29 keV). Left panel: In addition to iodine-125 in the thyroid, indium-111 in the lymph nodes are also imaged (red arrows). Right panel: The component from indium-111 is removed by our method. Credit: Yagishita et al.
Once the researchers were confident with their technique, they moved onto confirming it using mice. Three types of tracers with technetium-99m, Indium-111, and Iodine-125, respectively, were used in the experiment. These tracers accumulated in the lymph nodes and thyroid, respectively. Raw images showed noise and ghosting caused by radiation from other sources. However, by using the fitting technique, the researchers were able to identify unwanted radiation sources. When imaging iodine-125, only the thyroid gland where iodine-125 accumulates could be accurately and finely delineated by eliminating background noise and ghosting.
The researchers say there is still a way to go. While this study was an experiment involving a SPECT prototype, the researchers have already developed a full spec SPECT imaging device.
The team's method can be applied to biomedical research, radiopharmaceutical development, and clinical diagnostic techniques.
More information: Atsushi Yagishita et al, Simultaneous visualization of multiple radionuclides in vivo, Nature Biomedical Engineering (2022). DOI: 10.1038/s41551-022-00866-6
Journal information: Nature Biomedical Engineering
Provided by Kavli Institute for the Physics and Mathematics of the Universe