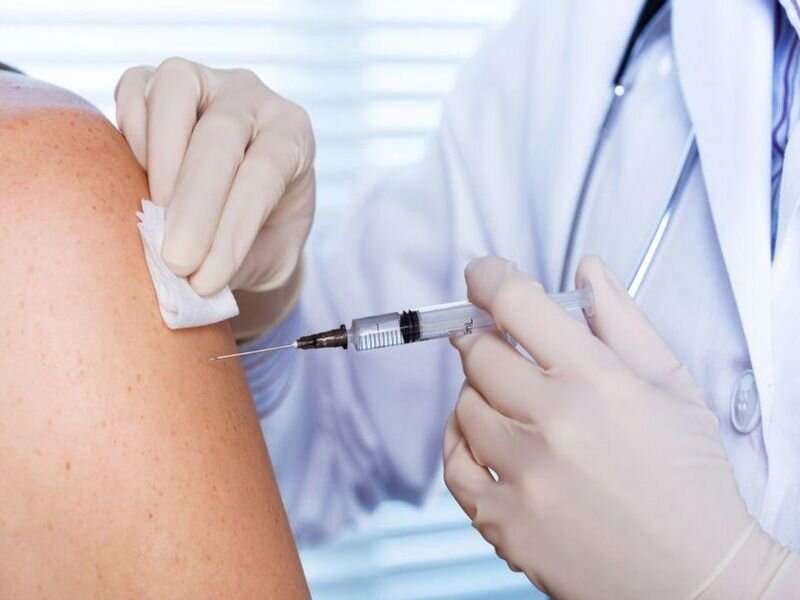
Hepatitis B (HepB) vaccination should be administered to adults aged 19 to 59 years and to those aged 60 years or older with risk factors for HepB, according to updated recommendations published in the April 1 issue of the U.S. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report.
Mark K. Weng, M.D., from the CDC in Atlanta, and colleagues updated recommendations for HepB vaccination for adults based on a review and discussion of the relevant scientific evidence. The review was informed by 263 studies deemed eligible.
The authors note that about one half of acute HepB cases reported in 2019 occurred among those aged 30 to 49 years and that the number of cases of acute HepB increased among adults aged 40 to 49 years and aged 50 to 59 years from 2011 to 2019. In addition, HepB vaccination coverage is low among adults aged 19 years and older. As a result, the Advisory Committee on Immunization Practices recommends that adults aged 19 to 59 years and those aged 60 years or older with risk factors should receive HepB vaccines, and that those aged 60 years or older without risk factors should be offered vaccination and may receive vaccines. Those who have completed a HepB vaccination series or with a history of HepB infection should not receive additional vaccination, apart from cases where revaccination may be indicated.
"Universal adult HepB vaccination through age 59 years removes the need for risk factor screening and disclosure and could increase vaccination coverage and decrease hepatitis B cases," the authors write.
More information: Mark K. Weng et al, Universal Hepatitis B Vaccination in Adults Aged 19–59 Years: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2022, MMWR. Morbidity and Mortality Weekly Report (2022). DOI: 10.15585/mmwr.mm7113a1
Two authors disclosed financial ties to the biopharmaceutical industry.
Journal information: Morbidity and Mortality Weekly Report
© 2022 HealthDay. All rights reserved.