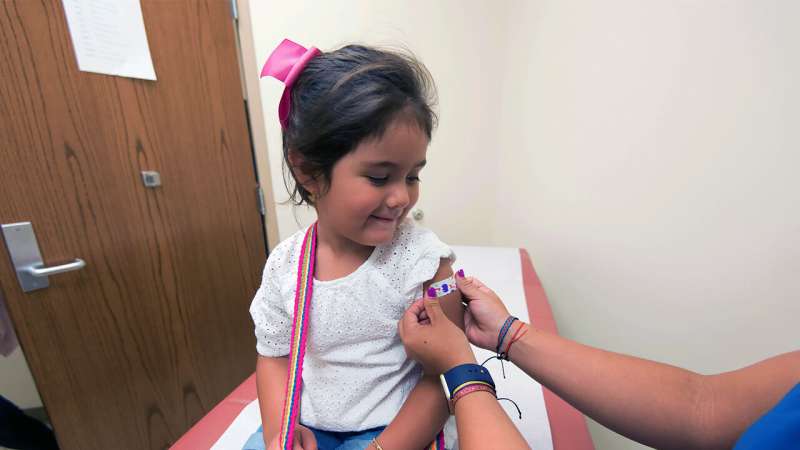
“We shouldn't rush with a one-size-fits-all approach, particularly when there is a product out there which would be of greater benefit to children,” says Dr Hamid Merchant. Credit: University of Huddersfield
Throughout the pandemic the University of Huddersfield's Department of Pharmacy has been raising awareness on what vaccines are, how they are formulated, and why they're an important part of the healthcare strategy as well as the progress on further developments in COVID vaccines, so that people can make an educated decision on becoming vaccinated or choosing vaccination for their children.
In response to the recent controversy about why COVID vaccines for children hadn't been approved in the UK but had in the US and why the UK was so slow to respond, the department's Dr. Hamid Merchant has written an article explaining why we should not rush mass-immunizing young children and how a delayed immunization can be beneficial in offering a more suitable vaccine formulation for children, such as the nasal COVID vaccine that should be approved soon.
The article, published in the Journal of Pharmaceutical Policy and Practice, agrees with the UK Government's Joint Committee on Vaccination and Immunization (JCVI) to not deliver a mandatory mass immunization program and explains why the current vaccines are not going to give the best outcomes in children in terms of efficacy and safety.
"Often scientist and public health professionals fear that discussing these issues openly may undermine vaccine uptake," said Dr. Merchant, "but, it's time that we explain the vaccine science and the differences between various vaccine formulations as a number of vaccine products are now approved by the regulatory agencies. We shouldn't rush with a one-size-fits-all approach, particularly when there is a product out there which would be of greater benefit to children."
The current situation
As matters currently stand the UK Government's Joint Committee on Vaccination and Immunization (JCVI) advises that COVID vaccines should be offered to 5–11-year-old children in the UK, but also deems such immunization as non-essential.
In the article, Dr. Merchant, who is a pharmaceutical scientist with over 19 years of experience in pharmaceutical research and development both in industry and academia, explains why the Committee was so conservative in approving COVID vaccines for children and why its official statement on the matter took so long to be released.
He also talks about how there are a number of alternatives for children that are very close to being approved and which have been specially designed to achieve the outcomes in children that current vaccines cannot deliver.
"We have tried to present a scientific explanation as to what vaccines are in general and how not every COVID vaccine is formulated the same," said Dr. Merchant.
"During the early stages of the immunization program there wasn't enough information available so by explaining how various vaccine formulations work and which ones are the safest considering an individual's pre-existing medical conditions (personalized medicines), we are enabling the public to become more educated when deciding what to do for themselves, or for their children."
As the vaccines have now done their job of enabling the country to live with the virus, Dr. Merchant believes it is imperative to continue investigating the next generation of COVID vaccines for all those who, in the future, still remain at high risk from emerging variants of concern.
"We have delivered a series of sessions and lectures for public and healthcare professionals alike to raise awareness of how various vaccines work, why they're a very important part of the healthcare strategy as well as the current progress in COVID vaccines development. This journal article is part of that campaign to increase medication safety amid a pandemic," he said.
More information: Hamid A. Merchant, Why COVID vaccines for young children (5–11 years) are not essential at this moment in time?, Journal of Pharmaceutical Policy and Practice (2022). DOI: 10.1186/s40545-022-00424-0
Provided by University of Huddersfield